Anesthesia Breathing Circuits Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 431844 | Date : Dec, 2025 | Pages : 255 | Region : Global | Publisher : MRU
Anesthesia Breathing Circuits Market Size
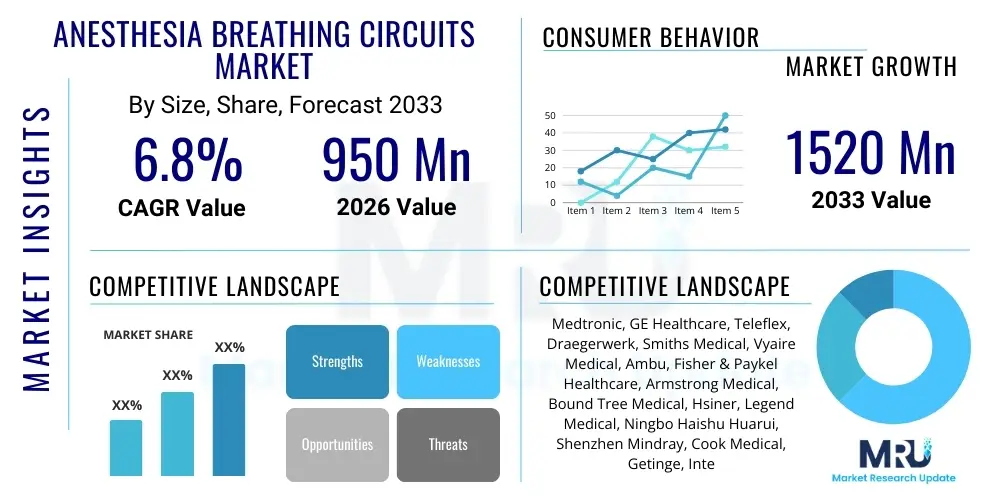
The Anesthesia Breathing Circuits Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2026 and 2033. The market is estimated at USD 950 million in 2026 and is projected to reach USD 1520 million by the end of the forecast period in 2033.
Anesthesia Breathing Circuits Market introduction
The Anesthesia Breathing Circuits Market encompasses devices and systems crucial for delivering anesthetic gases, oxygen, and facilitating the removal of carbon dioxide during surgical procedures. These circuits connect the patient's airway to the anesthesia machine, ensuring precise gas delivery and ventilation control. The primary components include breathing tubes, masks or endotracheal tubes, reservoirs (bags), and often specialized valves and carbon dioxide absorbers, depending on the circuit type used.
Key applications span a wide range of surgical disciplines, including general surgery, cardiology, neurosurgery, and orthopedics, particularly in hospital operating rooms and ambulatory surgical centers (ASCs). The shift toward disposable circuits has been a major trend, driven by stringent infection control standards and the need to minimize cross-contamination risk, thereby enhancing patient safety and streamlining workflow efficiency for clinical staff.
The market growth is primarily driven by the increasing global volume of surgical procedures, advancements in minimally invasive surgeries requiring specialized anesthetic protocols, and continuous innovation in circuit design focused on reducing dead space, improving gas flow dynamics, and enhancing overall patient monitoring capabilities during extended operations. Regulatory compliance and the push for standardized safety features are also significant driving factors influencing product development and adoption across various healthcare settings.
Anesthesia Breathing Circuits Market Executive Summary
The global Anesthesia Breathing Circuits Market is undergoing structural shifts characterized by a preference for advanced disposable systems and the integration of sophisticated monitoring technologies. Business trends indicate strong consolidation among key players who are focusing on vertically integrated supply chains and expanding their portfolios, particularly in highly regulated markets such as North America and Europe. The increasing prevalence of chronic diseases necessitating surgical interventions fuels demand, creating sustained revenue streams for manufacturers specializing in customizable and flexible circuit solutions optimized for different patient demographics, including pediatrics and geriatrics.
Regionally, North America maintains its dominance due to high healthcare expenditure, established infrastructure, and rapid adoption of premium disposable products. However, the Asia Pacific (APAC) region is emerging as the fastest-growing market segment, propelled by expanding healthcare access, rising surgical volume in developing economies like China and India, and significant government investments in modernizing hospital facilities. Regulatory harmonization efforts globally are also impacting market entry strategies, requiring manufacturers to meet diverse regional compliance standards simultaneously.
Segmentation trends highlight the increasing dominance of disposable circuits over reusable ones, driven primarily by safety mandates and operational efficiency gains in acute care settings. Furthermore, closed and semi-closed circuits are gaining traction due to their enhanced capabilities in conserving expensive anesthetic agents and reducing atmospheric pollution within the operating room. Technological advancements are increasingly focusing on integrating respiratory monitoring sensors directly into the circuit pathways to provide real-time data to anesthetists, thereby improving precision and responsiveness during critical perioperative phases.
AI Impact Analysis on Anesthesia Breathing Circuits Market
User inquiries regarding AI's influence typically revolve around how artificial intelligence can optimize gas delivery, predict patient physiological responses to anesthesia, and automate circuit management processes. Common questions address the integration of AI-driven predictive analytics for personalized anesthetic dosing, the role of machine learning in detecting subtle changes in ventilation patterns, and the potential for AI algorithms to manage closed-loop anesthesia delivery systems. Users express expectations for increased safety margins, reduced reliance on constant manual adjustments, and the creation of "smart" circuits that communicate real-time performance data to centralized operating room platforms. The core themes center on predictive maintenance, closed-loop feedback systems, and enhancing clinical decision support, suggesting a strong user interest in leveraging AI to move beyond passive monitoring toward proactive intervention.
- AI integration in closed-loop delivery systems for automated anesthetic gas titration, optimizing dosage based on real-time physiological data.
- Predictive maintenance analytics for early detection of circuit component degradation or failure, ensuring operational reliability.
- Enhanced decision support systems utilizing machine learning to predict patient movement, respiratory compromise, or adverse reactions related to circuit performance.
- Optimization of circuit material selection and design through generative AI, focusing on minimal flow resistance and reduced dead space.
- Improved workflow efficiency via AI-powered inventory management specific to disposable circuit variants and stock levels within surgical centers.
DRO & Impact Forces Of Anesthesia Breathing Circuits Market
The market dynamics for Anesthesia Breathing Circuits are shaped by a complex interplay of clinical necessity, technological innovation, and economic constraints. The primary drivers include the expanding elderly population, which requires more complex surgical care, and the substantial increase in the global volume of surgical procedures annually, particularly in fast-developing economies. Concurrently, the rigorous enforcement of infection control standards, favoring single-use disposable circuits, significantly propels market demand, overriding cost considerations in many high-income regions. These drivers are fundamentally linked to improving global healthcare access and safety protocols.
Key restraints, however, persist, notably the inherent complexity and high cost associated with advanced circuits integrated with monitoring accessories, which limits adoption in resource-limited settings. Furthermore, challenges related to the vast volumes of medical plastic waste generated by disposable circuits are increasingly scrutinized, pushing manufacturers toward developing more sustainable, biocompatible, or recyclable materials. These environmental concerns introduce long-term regulatory pressure that can slow down the overall growth trajectory of highly disposable segments.
Opportunities abound in emerging markets where healthcare infrastructure is rapidly developing and patient populations are vast and underserved. Technological advancements, especially in integrating circuits with sophisticated gas monitoring and low-flow anesthesia machines, present significant avenues for market penetration. The potential for developing smart, integrated circuits that minimize fresh gas usage not only reduces operational costs but also aligns with global environmental sustainability goals, offering a compelling value proposition to hospitals and ASCs seeking efficiency and green initiatives. The impact forces are generally high, driven by patient safety (a constant upward force) and moderated by cost sensitivity and waste management challenges.
Segmentation Analysis
The Anesthesia Breathing Circuits Market is comprehensively segmented based on product type, material composition, application area, and end-user base, reflecting the diverse requirements of modern anesthesia practice. The fundamental segmentation focuses on whether the circuits are reusable or disposable, with the latter currently dominating due to global infection control mandates. Further refinement occurs based on the mechanism of operation, specifically whether the circuit utilizes open, semi-open, semi-closed, or closed systems, each offering varying levels of rebreathing and gas conservation efficiency. This detailed segmentation allows manufacturers to tailor products precisely for specific clinical environments, ranging from high-volume university hospitals to specialized ambulatory surgical units.
- By Product Type:
- Open Circuits
- Semi-Open Circuits
- Semi-Closed Circuits
- Closed Circuits (High focus on low-flow anesthesia)
- Mapleson Systems (A, B, C, D/Bain, E, F/Jackson-Rees)
- By Component:
- Breathing Bags (Reservoir Bags)
- Breathing Tubes (Corrugated, Coaxial)
- Connectors and Adapters
- Filters (HME Filters, Viral/Bacterial Filters)
- Valves and APL Valves
- By Material:
- Disposable Circuits (PVC, PE, Silicone)
- Reusable Circuits (Silicone, Rubber)
- By End-User:
- Hospitals and Clinics (Largest segment)
- Ambulatory Surgical Centers (ASCs)
- Specialty Care Centers
- By Age Group:
- Adult Circuits
- Pediatric Circuits
- Neonatal Circuits
Value Chain Analysis For Anesthesia Breathing Circuits Market
The value chain for the Anesthesia Breathing Circuits Market is anchored by specialized raw material suppliers, primarily plastics, polymers (such as PVC and silicone for tubing), and specialized filter media. Upstream analysis involves the procurement and rigorous quality control of medical-grade materials, which must meet stringent biocompatibility and durability standards. Key upstream challenges include managing fluctuating commodity prices and ensuring a reliable supply of highly purified polymers necessary for sterile, disposable production. Manufacturers then undertake highly automated processes including extrusion, molding, assembly, sterilization (often using EtO or gamma radiation), and packaging in cleanroom environments.
The core distribution channels are bifurcated into direct sales models, particularly for large governmental or integrated health networks, and indirect distribution through established medical device distributors and wholesalers. These intermediaries play a critical role in inventory management, logistics, and providing regional market access, especially in fragmented healthcare systems. The choice between direct and indirect channels often hinges on the scale of the customer and the regional regulatory landscape, demanding specialized logistical handling due to the nature of sterile medical devices.
Downstream analysis focuses on the end-users—hospitals, ASCs, and clinics—who are the ultimate buyers. Their purchasing decisions are highly influenced by factors such as standardization across the facility, ease of integration with existing anesthesia machines, pricing structures (especially for high-volume disposable contracts), and clinical preference established by anesthetists. Marketing efforts are heavily focused on demonstrating superior patient safety features, cost-in-use benefits derived from reduced sterilization costs, and compatibility with low-flow anesthesia techniques, thereby justifying the initial product cost to procurement departments and clinical budget holders.
Anesthesia Breathing Circuits Market Potential Customers
The primary consumers and end-users of Anesthesia Breathing Circuits are institutions that perform surgical and critical care procedures requiring controlled mechanical ventilation and anesthetic gas delivery. Hospitals, particularly those with large operating room suites and intensive care units (ICUs), represent the largest segment of potential customers due to the high volume and complexity of their surgical schedules. These institutions often procure circuits under long-term contracts, prioritizing reliability, standardized safety features, and robust supply chain resilience, especially for disposable components that are critical consumables.
Ambulatory Surgical Centers (ASCs) constitute a rapidly growing customer base. ASCs primarily focus on outpatient surgeries and procedures, leading to a high demand for cost-effective, easy-to-use, and rapidly turn-around disposable circuits. Their purchasing criteria often emphasize efficiency and bulk pricing for frequently used standard circuits. Additionally, specialized critical care facilities and military field hospitals, which require durable and readily available ventilation support in emergency and non-traditional environments, also represent significant, albeit niche, potential customer segments demanding specific product features related to portability and environmental resilience.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 950 million |
| Market Forecast in 2033 | USD 1520 million |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Medtronic, GE Healthcare, Teleflex, Draegerwerk, Smiths Medical, Vyaire Medical, Ambu, Fisher & Paykel Healthcare, Armstrong Medical, Bound Tree Medical, Hsiner, Legend Medical, Ningbo Haishu Huarui, Shenzhen Mindray, Cook Medical, Getinge, Intersurgical, SunMed, Westmed |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Anesthesia Breathing Circuits Market Key Technology Landscape
The current technology landscape of the Anesthesia Breathing Circuits Market is defined by a shift towards integrated safety features and efficiency enhancements, particularly related to gas conservation. A critical technology involves advanced filtration systems, specifically Heat and Moisture Exchangers (HMEs) and high-efficiency particulate air (HEPA) filters, which are often integrated directly into disposable circuits to prevent cross-contamination, manage patient thermal regulation, and protect the anesthesia machine from moisture and particulate matter. The design focus is increasingly on reducing weight, maintaining flexibility without kinking, and ensuring standardized universal compatibility with a range of anesthesia ventilators and monitoring equipment across global platforms.
Furthermore, significant technological development is concentrated in coaxial circuits, such as the Bain system, which incorporate the inspiratory and expiratory limbs within a single outer tube. This design minimizes surface area and bulk, reducing the physical presence in the operating room while maintaining gas dynamics. Coupled with this is the continuous refinement of low-flow and minimal-flow anesthesia techniques, driving demand for specialized closed circuits that maximize rebreathing efficiency and necessitate less consumption of costly volatile anesthetic agents. Innovations in material science are also crucial, exploring non-PVC polymers that offer superior handling characteristics and reduced environmental impact.
The emerging technological frontier involves the incorporation of miniaturized electronic sensors directly within the circuit components. These sensors facilitate real-time monitoring of pressure, flow, temperature, and end-tidal CO2 (ETCO2), enabling a higher degree of precision and integration with electronic health records and specialized anesthetic information systems (AIMS). The ultimate goal is to create smart, sensor-enabled circuits that function as an active part of a patient monitoring network, providing clinicians with immediate feedback necessary for executing complex anesthetic plans, positioning the market for eventual transition toward fully automated, closed-loop delivery systems leveraging AI algorithms.
Regional Highlights
The geographical distribution of the Anesthesia Breathing Circuits Market reflects varied healthcare maturity levels, surgical volumes, and regulatory standards regarding infection control and medical device approval. North America (NA) commands the largest market share, characterized by high penetration rates of disposable circuits, advanced healthcare infrastructure, and significant expenditure on sophisticated medical technology. The rigorous infection prevention standards set by institutions and regulatory bodies in the US and Canada accelerate the adoption of premium, single-use products, despite their higher initial cost. Innovation adoption, including integrated monitoring circuits, is typically fastest in this region, setting global trends for others to follow.
Europe represents the second-largest market, marked by stringent European Union medical device regulations (MDR) that necessitate high quality and safety standards. Western European countries exhibit high demand for high-efficiency circuits suitable for low-flow anesthesia, driven by environmental consciousness and cost-saving mandates regarding expensive volatile agents. Germany, the UK, and France are crucial markets, demonstrating a balanced mix of domestic manufacturing and imports, with pricing pressures often managed through centralized purchasing schemes or national health service guidelines.
Asia Pacific (APAC) is projected to record the highest growth rate during the forecast period. This rapid expansion is primarily fueled by massive infrastructure investments in emerging economies like China, India, and Southeast Asian nations, leading to increased surgical capacity and improved healthcare access for large populations. While reusable circuits still hold a notable share in price-sensitive segments, the rising awareness of surgical safety and the influence of multinational companies are quickly shifting preferences toward disposable solutions, particularly in large urban centers and private hospital networks.
Latin America (LATAM) and the Middle East & Africa (MEA) markets present unique challenges and opportunities. LATAM growth is steady, influenced heavily by US and European regulatory standards and product availability, though economic volatility and centralized budget constraints often impact purchasing power. The MEA region, particularly the Gulf Cooperation Council (GCC) countries, demonstrates a high demand for state-of-the-art equipment driven by government-funded modern healthcare projects, mirroring the advanced adoption patterns seen in North America but constrained by localized logistical complexities and reliance on imported products.
- North America: Market leader; characterized by high disposable circuit adoption, advanced monitoring integration, and stringent regulatory environment. High surgical throughput in hospitals and ASCs.
- Europe: Strong focus on low-flow anesthesia compatible closed and semi-closed circuits; driven by EU MDR compliance and environmental efficiency mandates. Steady growth rate.
- Asia Pacific (APAC): Fastest growing region; massive expansion in healthcare infrastructure, increasing surgical volume, and gradual transition from reusable to disposable systems in major economic hubs.
- Latin America (LATAM): Growth driven by increasing access to elective procedures and modernization of hospital facilities, though sensitive to macroeconomic factors and currency fluctuations.
- Middle East and Africa (MEA): Premium market segments in GCC nations driven by government investment in world-class hospitals; demand for high-quality imported devices; varied adoption rates across the African continent.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Anesthesia Breathing Circuits Market.- Medtronic plc
- GE Healthcare (A Segment of General Electric Company)
- Teleflex Incorporated
- Draegerwerk AG & Co. KGaA
- Smiths Medical (Part of ICU Medical)
- Vyaire Medical, Inc.
- Ambu A/S
- Fisher & Paykel Healthcare Corporation Limited
- Armstrong Medical Ltd.
- Bound Tree Medical LLC (A Sarnova Company)
- Hsiner Co., Ltd.
- Legend Medical Devices, Inc.
- Ningbo Haishu Huarui Medical Device Co., Ltd.
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
- Cook Medical LLC
- Getinge AB
- Intersurgical Ltd.
- SunMed, LLC
- Westmed, Inc.
- Becton, Dickinson and Company (BD)
Frequently Asked Questions
Analyze common user questions about the Anesthesia Breathing Circuits market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is driving the market shift toward disposable Anesthesia Breathing Circuits?
The primary driver is the necessity for stringent infection control and patient safety protocols, reducing the risk of cross-contamination and eliminating the high operational costs and workflow complexities associated with reusable device sterilization and reprocessing.
How do technological advancements influence the efficiency of anesthetic gas usage?
Technological advancements, particularly the development of closed and semi-closed circuits, along with high-efficiency HME filters and integrated monitoring sensors, allow for the use of low-flow anesthesia, significantly reducing the consumption of expensive volatile anesthetic agents and improving economic efficiency.
Which geographical region exhibits the fastest growth potential for Anesthesia Breathing Circuits?
The Asia Pacific (APAC) region is projected to demonstrate the highest Compound Annual Growth Rate (CAGR), driven by large-scale investments in healthcare infrastructure, expanding surgical capacity, and increasing adoption of modern disposable medical consumables in countries like China and India.
What role does AI play in the future development of Anesthesia Breathing Circuits?
AI is expected to enable the next generation of smart circuits by facilitating closed-loop anesthesia delivery, using predictive analytics for personalized gas dosing, and providing enhanced real-time decision support systems to improve surgical safety and anesthetic precision.
What are the key differences between Mapleson D and Mapleson A circuits?
The Mapleson A circuit (Magill) is highly efficient for spontaneous breathing patients but requires higher fresh gas flows for controlled ventilation. Conversely, the Mapleson D circuit (Bain) is more efficient for controlled ventilation, is less bulky due to its coaxial design, and is widely used for pediatric applications and during transport.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
