Aseptic Sampling System Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 433384 | Date : Dec, 2025 | Pages : 255 | Region : Global | Publisher : MRU
Aseptic Sampling System Market Size
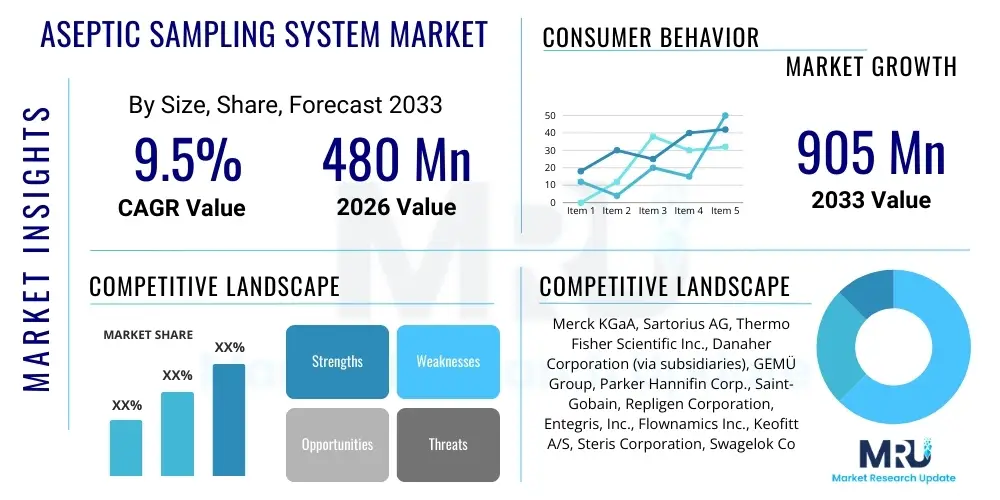
The Aseptic Sampling System Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 9.5% between 2026 and 2033. The market is estimated at USD 480 million in 2026 and is projected to reach USD 905 million by the end of the forecast period in 2033.
The robust growth trajectory is primarily driven by the escalating demand for biopharmaceuticals, including monoclonal antibodies and vaccines, which necessitate stringent quality control and contamination prevention throughout the manufacturing lifecycle. Aseptic sampling systems are critical components in maintaining the sterility of process fluids, ensuring regulatory compliance (such as FDA cGMP guidelines), and safeguarding patient safety. The shift from traditional manual sampling methods, which introduce significant contamination risk, towards automated, closed-loop systems is accelerating market expansion.
Furthermore, continuous manufacturing initiatives within the pharmaceutical sector are increasing the requirement for reliable, in-line, and at-line sampling solutions that can integrate seamlessly with automated process analytical technology (PAT) tools. This integration allows for real-time monitoring of critical quality attributes (CQAs), thereby reducing batch failure rates and improving overall process efficiency. Geographical expansion in emerging economies, particularly in the Asia Pacific region, characterized by increasing investment in biotech infrastructure and CMO/CDMO capacity, is set to provide significant tailwinds to the Aseptic Sampling System Market over the forecast period.
Aseptic Sampling System Market introduction
The Aseptic Sampling System Market encompasses specialized equipment designed to extract representative samples from a bioprocess or pharmaceutical manufacturing environment without compromising the sterility of either the sample or the bulk production stream. These systems utilize closed-loop mechanisms, often incorporating sterile connectors, diaphragm valves, single-use bags, or specialized bottles, to prevent external contaminants from entering the system. Major applications span sterile drug substance manufacturing, final product filling, media preparation, fermentation/cell culture monitoring, and purified water loop validation, serving the critical function of ensuring product safety and efficacy as required by global regulatory bodies. Key benefits include enhanced sample integrity, reduced risk of microbial ingress, improved operator safety by limiting exposure to potent compounds, and greater operational efficiency through automation. The market is fundamentally driven by the rapid growth of the biotechnology sector, stringent global regulatory enforcement regarding process validation, and the industry-wide transition towards continuous and automated bioprocessing workflows.
Aseptic Sampling System Market Executive Summary
The Aseptic Sampling System Market is characterized by a strong transition towards single-use technology (SUT) driven by flexibility, reduced validation costs, and minimized cross-contamination risk, establishing SUT as a dominant business trend, particularly in the production of high-value biologics and personalized medicines. Regionally, North America maintains market leadership due to substantial R&D investment, a mature biopharmaceutical ecosystem, and the presence of major system integrators and technology pioneers, while the Asia Pacific region is demonstrating the highest growth velocity, fueled by increasing government support for domestic vaccine and biopharma manufacturing capabilities, notably in China and India. Segment-wise, the Manual/Semi-Automated systems still hold a substantial share but are rapidly yielding ground to Fully Automated systems, which offer superior reproducibility and data traceability essential for modern Quality by Design (QbD) approaches; furthermore, the Biopharmaceutical segment, specifically focused on upstream cell culture and downstream purification, remains the largest end-user category due to the inherent complexity and sterility requirements of biologic drug production.
AI Impact Analysis on Aseptic Sampling System Market
Common user questions regarding AI's influence on the Aseptic Sampling System Market center around its capability to enhance predictive maintenance, optimize sampling frequency, ensure real-time data integrity, and automate decision-making processes regarding process deviation. Users are keenly interested in how AI algorithms can analyze complex process analytical technology (PAT) data streams, collected during automated aseptic sampling, to predict potential batch contamination events or material failure (such as membrane breaches or filter clogging) before they occur, thereby moving quality control from reactive testing to proactive assurance. The key expectation is that AI integration will transform aseptic sampling from a necessary compliance step into a predictive process optimization tool, fundamentally reducing human intervention, minimizing false positives, and decreasing the total cycle time for batch release through superior data evaluation and anomaly detection capabilities.
- AI enables predictive maintenance of sampling system components (e.g., pumps, seals, valves) based on operational data analysis.
- Machine learning optimizes sampling schedules and locations by correlating sample results with real-time process parameters (temperature, pH, turbidity).
- AI algorithms facilitate real-time anomaly detection in sample integrity, instantly flagging potential contamination or system breaches.
- Integration with LIMS and MES systems ensures automated data capture, analysis, and generation of regulatory compliance reports, improving traceability.
- Neural networks are used to interpret complex spectroscopic data (e.g., Raman, NIR) collected via in-line sampling for continuous quality verification.
DRO & Impact Forces Of Aseptic Sampling System Market
The Aseptic Sampling System Market is significantly shaped by a confluence of driving, restraining, and opportunity forces. Key drivers include the exponential expansion of the biopharma sector, particularly the production of advanced therapies and vaccines requiring strict sterility assurance, alongside stringent global regulatory mandates (FDA, EMA) promoting closed-loop systems to minimize environmental exposure and human error. Restraints primarily involve the high initial capital investment required for implementing sophisticated automated systems, particularly in smaller facilities or emerging markets, coupled with the complexity associated with system validation and integration into existing legacy infrastructure. Opportunities are abundant, rooted in the ongoing technological evolution towards single-use aseptic assemblies that simplify setup and cleaning, and the growing industry focus on continuous biomanufacturing which mandates reliable, automated, and continuous sampling integrated with Process Analytical Technology (PAT), offering enhanced efficiency and reduced time-to-market. These forces collectively dictate the strategic trajectory of the market, pushing manufacturers towards disposable, standardized, and highly automated solutions that promise enhanced compliance and operational flexibility.
Segmentation Analysis
The Aseptic Sampling System Market is structurally segmented based on product type, application, system type, and end-user, reflecting the diverse requirements across the pharmaceutical and biotechnology industries. Product type segmentation distinguishes between various hardware components like bottles, connectors, and valves versus complete automated systems. Application segments delineate use across bioprocessing (upstream and downstream), formulation, and regulatory quality control. The classification by system type, separating manual, semi-automated, and fully automated platforms, highlights the ongoing industry transition towards higher levels of automation necessary for reproducibility and data integrity. End-user classification confirms the dominance of biopharmaceutical manufacturers and Contract Manufacturing Organizations (CMOs) due to their intensive need for high-sterility monitoring protocols and large-scale validation projects.
- By Product Type:
- Bottles and Bags
- Connectors and Couplings
- Valves (Diaphragm, Ball, Radial)
- Accessories and Components (Tubing, Manifolds)
- Automated Systems/Skids
- By System Type:
- Manual/Semi-Automated Systems
- Fully Automated Systems
- By Application:
- Upstream Bioprocessing (Fermentation/Cell Culture)
- Downstream Bioprocessing (Purification)
- Aseptic Fill/Finish
- Sterile API Manufacturing
- Quality Control and Assurance
- By End-User:
- Biopharmaceutical and Pharmaceutical Companies
- Contract Manufacturing Organizations (CMOs) and Contract Development and Manufacturing Organizations (CDMOs)
- Academic and Research Institutes
Value Chain Analysis For Aseptic Sampling System Market
The value chain for the Aseptic Sampling System Market begins with the Upstream Analysis, which focuses on the procurement of high-grade raw materials essential for maintaining sterility, including certified USP Class VI plastics (for single-use components), specialized stainless steel (316L for reusable systems), and precision electronic components for automation modules. Raw material suppliers must adhere to strict quality standards as the integrity of the final sampling system relies heavily on material purity and compatibility with potent chemicals and biological media. This phase also includes component manufacturing, where specialized fabrication of sterile valves, proprietary connectors, and customized tubing assemblies takes place under strict cleanroom conditions, emphasizing precision engineering to eliminate potential dead legs or areas where microbial growth could occur.
The midstream segment involves the core manufacturing and assembly of the integrated sampling systems, where manufacturers combine components into final single-use manifolds or permanent, customizable skids. Crucially, the quality assurance and testing phase, including gamma irradiation or autoclave validation for single-use products, adds significant value here. Distribution Channel analysis reveals a mixture of Direct and Indirect sales models. High-volume, standardized single-use assemblies are often distributed indirectly through global medical supply distributors and regional partners, benefiting from their established logistics networks. Conversely, highly complex, customized automated sampling skids or permanent systems usually necessitate a Direct sales approach, involving extensive technical consultation, Factory Acceptance Testing (FAT), and site-specific validation services provided directly by the original equipment manufacturer (OEM) or specialized system integrators.
The Downstream Analysis culminates with the end-users—primarily large biopharmaceutical companies and CDMOs—where the systems are integrated into critical manufacturing processes. Value creation at this stage involves installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) validation services, often provided by the OEM to ensure regulatory compliance and seamless workflow integration. Post-sales support, including maintenance, calibration, and provision of certified spare parts and replacement single-use components, is paramount. The value chain is constantly being optimized towards reducing lead times for customized single-use assemblies and improving data management integration capabilities at the end-user level, reflecting the critical, non-negotiable role these systems play in modern sterile drug manufacturing.
Aseptic Sampling System Market Potential Customers
The primary end-users and potential buyers of Aseptic Sampling Systems are organizations operating in environments where maintaining absolute sterility is mandatory for product safety and regulatory adherence, specifically dominated by the Biopharmaceutical and Pharmaceutical manufacturing sectors. These customers require highly reliable, validated systems for monitoring critical parameters during both upstream (fermentation and cell culture) and downstream (purification and filtration) processes, ensuring samples accurately reflect the status of the bulk product without introducing contaminants. The increasing global reliance on Contract Development and Manufacturing Organizations (CDMOs) makes them a significant and rapidly growing customer segment, as CDMOs must invest in flexible, fast-turnaround aseptic sampling solutions, particularly single-use formats, to support a diverse portfolio of client projects and varying scales of operation, often necessitating quick changeovers and minimal cleaning validation time.
Furthermore, specialized segments such as producers of Advanced Therapy Medicinal Products (ATMPs), including gene and cell therapies, represent high-value potential customers. These therapies are often manufactured at a smaller scale but require extremely strict, almost zero-tolerance, levels of contamination control due to their unique biological nature and regulatory scrutiny. Academic and large-scale industrial research laboratories involved in drug discovery, process development, and scale-up studies also form a steady customer base, utilizing these systems to validate new process designs before transferring them to commercial manufacturing. All these customer segments prioritize systems that offer superior documentation, robust automation capabilities, and ease of integration with existing digital quality management systems (QMS), making traceability and compliance key purchasing criteria.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 480 million |
| Market Forecast in 2033 | USD 905 million |
| Growth Rate | 9.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Merck KGaA, Sartorius AG, Thermo Fisher Scientific Inc., Danaher Corporation (via subsidiaries), GEMÜ Group, Parker Hannifin Corp., Saint-Gobain, Repligen Corporation, Entegris, Inc., Flownamics Inc., Keofitt A/S, Steris Corporation, Swagelok Company, Dover Corporation, DrM, Dr. Mueller AG, Marchesini Group, Wipotec Group, Esco Group, Finesse Solutions (part of Thermo Fisher), Colder Products Company (CPC) |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Aseptic Sampling System Market Key Technology Landscape
The technological landscape of the Aseptic Sampling System Market is rapidly evolving, driven primarily by the need for increased standardization, enhanced automation, and elimination of cross-contamination risks through disposable components. The core technology centers around specialized valve designs, particularly zero dead leg diaphragm valves and radial diaphragm valves, which ensure complete drainage and sterilization capabilities, minimizing areas where microbial growth can proliferate. A major technological shift is the widespread adoption of Single-Use Technology (SUT), manifesting in pre-sterilized, disposable manifold assemblies that integrate tubing, connectors (such as quick-connect sterile coupling devices), and sample bottles or bags. These SUT solutions eliminate the lengthy and costly Clean-In-Place (CIP) and Steam-In-Place (SIP) validation cycles required for stainless steel systems, significantly increasing operational throughput and flexibility, especially for multi-product facilities like CDMOs.
Automation and integration with Process Analytical Technology (PAT) represent the cutting edge of current technological development. Fully automated aseptic sampling skids incorporate precision peristaltic pumps, automated pinch valves, and sophisticated control systems (often running on PLC or distributed control systems) that manage timed sampling events, ensuring highly repeatable volumes and minimizing manual intervention. Furthermore, the integration allows for samples to be automatically delivered to on-line or at-line analytical instruments (e.g., cell counters, osmometers, chromatography systems) while maintaining the closed system integrity. Future technology is focusing on microfluidic sampling platforms and advanced sensor integration directly within the sterile path, enabling continuous, real-time sampling and analysis, thus moving the industry closer to truly autonomous biomanufacturing processes and dramatically enhancing Quality by Design (QbD) principles.
Regional Highlights
- North America: Dominates the global market share due to the highest concentration of leading biopharmaceutical companies, robust investment in advanced drug discovery and development, and early adoption of closed and automated sampling technologies. Strict FDA regulatory oversight regarding validation and sterility mandates the use of cutting-edge aseptic systems, particularly in the production of blockbuster biologics and emerging cell and gene therapies.
- Europe: Represents a mature market characterized by strong regulatory compliance through the European Medicines Agency (EMA) and significant governmental focus on vaccine production. Western European countries, particularly Germany, Switzerland, and Ireland, are major manufacturing hubs, driving demand for high-quality, validated stainless steel and advanced single-use sampling solutions integrated into complex bioreactor systems.
- Asia Pacific (APAC): Expected to exhibit the highest Compound Annual Growth Rate (CAGR) globally, primarily driven by rapid expansion of domestic pharmaceutical manufacturing in China, India, and South Korea, coupled with significant foreign direct investment. Governments are actively supporting the localization of vaccine production and biopharma capacity, generating massive demand for cost-effective, yet compliant, aseptic sampling systems, favoring rapid deployment of single-use assemblies.
- Latin America (LATAM): A developing market facing increasing pressure to align with international cGMP standards. Growth is steady, focused mainly on importing validated systems to support localized production of generic sterile injectable drugs and vaccines. Market penetration is often linked to the regional establishment of international pharma facilities.
- Middle East and Africa (MEA): A nascent market with growth concentrated in technologically advanced nations like Israel, Saudi Arabia, and the UAE, which are building modern biotech infrastructure. The adoption rate of aseptic sampling is accelerating, particularly for vaccine production and sterile compounding, though procurement remains reliant on global suppliers and integrators.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Aseptic Sampling System Market.- Merck KGaA
- Sartorius AG
- Thermo Fisher Scientific Inc.
- Danaher Corporation (including key life science subsidiaries)
- GEMÜ Group
- Parker Hannifin Corp.
- Saint-Gobain
- Repligen Corporation
- Entegris, Inc.
- Flownamics Inc.
- Keofitt A/S
- Steris Corporation
- Swagelok Company
- Dover Corporation
- DrM, Dr. Mueller AG
- Marchesini Group
- Wipotec Group
- Esco Group
- Finesse Solutions (part of Thermo Fisher)
- Colder Products Company (CPC)
Frequently Asked Questions
Analyze common user questions about the Aseptic Sampling System market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary difference between traditional and aseptic sampling systems?
Traditional sampling systems often involve manual opening of the process line to extract a sample, creating a risk of environmental contamination for both the sample and the product stream. Aseptic sampling systems, conversely, use closed-loop designs, often incorporating sterile membranes or specialized valves (like diaphragm valves) and disposable components, ensuring that the sample is collected without exposing the bulk product to the outside environment, thus maintaining absolute sterility and regulatory compliance.
How does Single-Use Technology (SUT) impact the Aseptic Sampling Market?
SUT has fundamentally shifted the market by offering pre-sterilized, disposable sampling manifolds and connectors, eliminating the necessity for time-consuming and expensive Clean-In-Place (CIP) and Steam-In-Place (SIP) validation cycles. This significantly reduces turnaround time between batches, minimizes cross-contamination risk, and lowers overall operational costs, making SUT the preferred choice for flexible biomanufacturing and Contract Development and Manufacturing Organizations (CDMOs).
Which end-user segment drives the highest demand for automated aseptic sampling systems?
The Biopharmaceutical segment, particularly manufacturers involved in large-scale production of monoclonal antibodies (mAbs) and vaccines, drives the highest demand. These products require intensive monitoring during upstream cell culture and downstream purification, necessitating fully automated systems that provide highly reproducible samples and integrate seamlessly with Process Analytical Technology (PAT) for continuous quality verification and compliance with cGMP standards.
What are the main regulatory challenges facing the adoption of new aseptic sampling technologies?
The main challenges involve the rigorous qualification and validation requirements mandated by bodies like the FDA and EMA. New technologies, especially custom single-use assemblies or complex automated skids, require extensive validation protocols (IQ/OQ/PQ) to prove they maintain sterility and sample integrity consistently. Furthermore, ensuring that all components meet USP Class VI standards and that the system design prevents "dead legs" or fluid stagnation is critical for regulatory approval.
How is Process Analytical Technology (PAT) integrated with modern aseptic sampling solutions?
Modern aseptic sampling systems are designed to be integral components of PAT initiatives. Automated systems can collect samples at precise, programmed intervals and deliver them directly to at-line analytical instruments (such as chromatographs or biosensors) while maintaining sterility. This integration allows for real-time monitoring of Critical Quality Attributes (CQAs), facilitating rapid process adjustments, reducing reliance on slow, off-line QC labs, and enabling true continuous manufacturing.
The detailed analysis within this report underscores the market’s reliance on technological advancement and stringent regulatory adherence. Growth will be concentrated in systems that offer superior automation, single-use flexibility, and robust data integrity features, crucial for the future landscape of high-purity biomanufacturing. Strategic partnerships between hardware manufacturers and software providers specializing in bioprocess data management will be key differentiators in the highly competitive aseptic sampling ecosystem, ensuring that systems not only maintain sterility but also contribute actionable data for process optimization.
The global shift towards personalized medicine and smaller batch sizes further emphasizes the value of flexible, quick-changeover aseptic sampling solutions. While North America and Europe continue to lead in terms of technology adoption and market value, the accelerating investment in biopharma capacity across the Asia Pacific region ensures that this area remains the epicenter of future market growth. Companies that can standardize their SUT products and provide comprehensive validation support tailored to regional regulatory requirements are best positioned to capture this expanding demand and solidify their leadership within the Aseptic Sampling System Market over the forecast period.
In essence, the future market trajectory is inextricably linked to the industry's commitment to continuous manufacturing principles and digital transformation. Automated aseptic systems are pivotal to this transformation, acting as the critical link between the sterile production environment and the data-driven quality control necessary for fast, compliant, and efficient production of life-saving biologics. The complexity of new therapies demands zero-tolerance for contamination, solidifying the Aseptic Sampling System as a mission-critical investment for global pharmaceutical entities.
The adoption of advanced materials science is also playing a significant role. Manufacturers are continually researching and integrating proprietary polymers and specialized elastomer blends into sterile connectors and tubing to improve chemical compatibility, minimize extractables and leachables (E&L), and enhance the physical resilience of single-use components, which is crucial when handling high-temperature sterilization processes or aggressive solvent media often used in downstream purification. Ensuring long-term component stability under diverse bioprocessing conditions remains a core focus area for R&D investment.
Another emerging trend is the development of modular and configurable aseptic sampling skids. Instead of rigid, fixed systems, end-users increasingly demand systems that can be rapidly reconfigured to accommodate different process scales or drug products. This modularity not only aids in accelerating process development timelines but also offers significant flexibility to Contract Manufacturing Organizations (CMOs) dealing with fluctuating production schedules and varied client specifications. This demand pushes manufacturers to engineer standardized interfaces and universal components that can interoperate seamlessly, streamlining the validation process for diverse production setups.
Furthermore, the digitalization of aseptic sampling workflows includes the implementation of electronic batch records (EBR) integration. Automated systems not only collect the sample but also record all metadata associated with the sampling event—time, date, pressure, flow rate, and operator ID—directly into the plant's Manufacturing Execution System (MES) or Laboratory Information Management System (LIMS). This automated documentation minimizes human error in data transcription, strengthens data integrity, and significantly speeds up the review-by-exception process for final batch release, offering a substantial competitive advantage to manufacturers adopting these advanced capabilities.
The sustainability aspect is also beginning to influence purchasing decisions, particularly in Europe. While single-use technology inherently generates plastic waste, manufacturers are addressing this by exploring material options that are easier to recycle or by developing lighter, more efficient designs that reduce the overall plastic footprint per batch. Although stainless steel systems offer inherent reusability, their high utility consumption for CIP/SIP cycles makes single-use assemblies, when properly managed through specialized recycling programs, increasingly competitive from a total environmental footprint perspective.
Geopolitical factors, particularly supply chain resilience, are impacting the market structure. The reliance on global suppliers for specialized components (like USP Class VI certified polymers and proprietary connectors) has prompted biopharma companies to seek dual-sourcing strategies or regional manufacturing hubs for aseptic components. This emphasis on regional self-sufficiency is driving local component manufacturing investments, particularly in high-growth regions like APAC, mitigating risks associated with international logistics and trade uncertainties.
The high barrier to entry for new competitors in the Aseptic Sampling Market is maintained by the necessity of extensive regulatory approvals and the requirement for proven reliability and robustness. Customers prioritize established suppliers with a long history of successful system validation and comprehensive technical support, recognizing that failure of an aseptic component can lead to entire batch losses worth millions of dollars. Therefore, brand trust, comprehensive documentation, and deep application expertise remain critical determinants of market success, favoring incumbent market leaders.
Lastly, training and skilled labor availability represent a latent challenge. While automation reduces manual error, it necessitates personnel trained not only in bioprocessing but also in sophisticated instrument operation, maintenance, and data interpretation (including basic AI/ML model understanding for predictive maintenance). Manufacturers are addressing this by offering extensive training programs and user-friendly interfaces, aiming to simplify complex operational procedures and ease the integration of highly advanced aseptic sampling technologies into the existing workforce skillset.
The market's sustained expansion is assured by the foundational shift in pharmaceutical manufacturing towards sterile biologics, coupled with an unyielding global regulatory environment that continuously raises the bar for quality assurance. Companies leveraging integration capabilities—linking aseptic sampling data directly into enterprise resource planning (ERP) and QMS systems—are driving the industry standard, ensuring that the process of obtaining a small, representative sample remains the most reliable indicator of overall batch quality and safety. The continuous innovation in sterile connectivity and fluid management components ensures that system downtime related to sampling processes is minimized, directly contributing to higher manufacturing capacity utilization across the pharmaceutical value chain.
Future growth will also be fueled by the expansion of the cell and gene therapy sector. These therapies, often manufactured in small, highly customized batches, demand incredibly precise and closed sampling methodologies to preserve viability and prevent contamination of delicate cellular material. Aseptic sampling systems tailored for low-volume, high-value cell culture applications, potentially incorporating microfluidics for minimal sample loss, represent a specialized, high-growth niche within the overall market landscape. This focus on precision and minimization of waste in high-cost environments highlights the strategic importance of reliable, non-contaminating sample acquisition.
In summary, the Aseptic Sampling System Market is characterized by accelerating technological advancements in SUT and automation, driven by stringent regulatory pressures and the rapid growth of the biopharma sector. Success hinges on manufacturers' ability to provide validated, integrated, and flexible solutions that meet the evolving demands of continuous manufacturing and high-purity applications, securing the integrity of critical processes from upstream media preparation through to final fill/finish.
The shift towards continuous bioprocessing (CB) is particularly impactful, as CB requires continuous, real-time data input, which manual or semi-automated sampling cannot reliably provide. Fully automated aseptic sampling systems integrated with advanced analytical instruments (PAT) enable manufacturers to maintain a steady state of quality control, minimizing process variance and ensuring compliance with emerging regulatory guidance that favors real-time release testing over traditional end-product testing. This paradigm shift makes the investment in automated aseptic systems a necessity rather than an option for large-scale, modern biomanufacturing facilities seeking operational excellence and maximized yield.
Moreover, standardization within the single-use connector segment is a key development. While proprietary connectors ensure performance for specific systems, the industry is moving towards more open-source or widely adopted sterile connection standards (like genderless connectors) to enhance supply chain flexibility and reduce compatibility issues when integrating components from multiple vendors. This standardization benefits end-users by simplifying inventory management and accelerating the design and validation of complex single-use manifold assemblies, further democratizing the access to high-quality aseptic sampling solutions.
Finally, cybersecurity considerations are becoming increasingly important, particularly for highly automated, network-connected sampling skids. As systems integrate with MES and ERP platforms, securing the control software and data transmission against unauthorized access or manipulation is vital to maintaining data integrity and regulatory compliance. Manufacturers are now incorporating hardened security protocols and encryption standards into their automation platforms to address these emerging threats, ensuring that the critical quality data generated by the aseptic sampling process remains trustworthy and inviolable throughout the product lifecycle.
The robustness of the market is fundamentally supported by the non-negotiable requirement for sterility in pharmaceutical and biological production, making aseptic sampling systems critical capital expenditure. The confluence of demographic changes, increased prevalence of chronic diseases requiring biologic treatments, and global preparedness for pandemic threats (requiring rapid vaccine production) ensures a sustained high demand for these essential quality control tools well into the next decade. The competitive landscape focuses intensely on innovation in disposables, miniaturization, and data integration capabilities, driving a cycle of continuous improvement in performance and compliance features.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
