Bacteriophage Therapy Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 437359 | Date : Dec, 2025 | Pages : 258 | Region : Global | Publisher : MRU
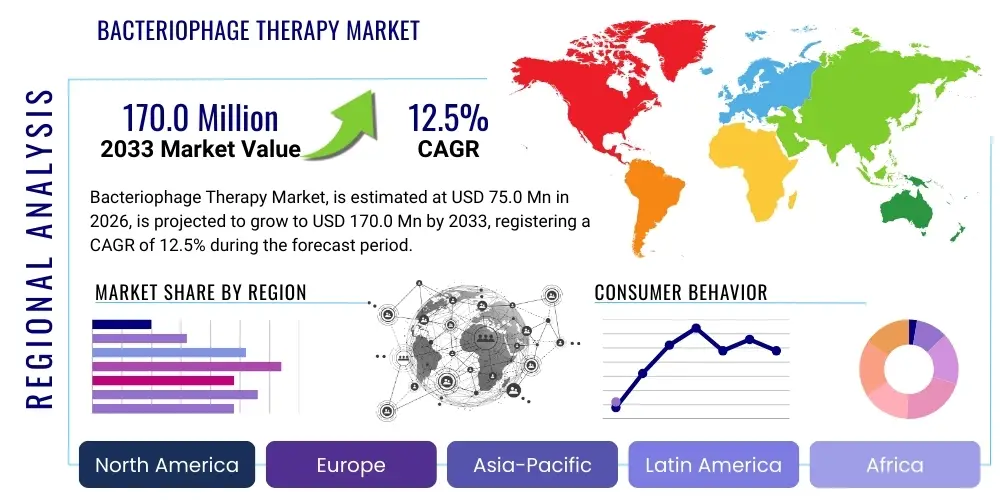
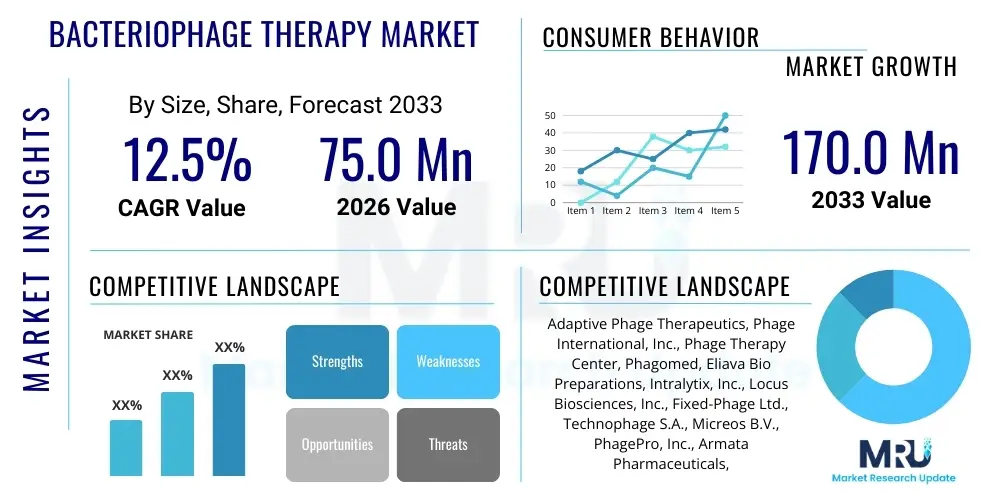
Bacteriophage Therapy Market Size
The Bacteriophage Therapy Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 12.5% between 2026 and 2033. The market is estimated at USD 75.0 million in 2026 and is projected to reach USD 170.0 million by the end of the forecast period in 2033.
Bacteriophage Therapy Market introduction
Bacteriophage therapy represents a highly specialized and increasingly relevant field of personalized medicine, utilizing naturally occurring viruses (bacteriophages, or phages) to specifically target and destroy pathogenic bacteria. This approach is gaining significant traction globally, primarily driven by the escalating crisis of Antimicrobial Resistance (AMR), which conventional antibiotics are failing to address effectively. Phages offer a distinct advantage over broad-spectrum antibiotics because they possess high specificity, minimizing disruption to the host's beneficial microbiome, thereby reducing common side effects associated with traditional antimicrobial treatments. The therapy’s application spans critical areas, including chronic infections, orthopedic infections, and severe cases of sepsis where standard treatments have been exhausted.
The core mechanism involves the selection and administration of lytic phages that infect the target bacteria, replicate within them, and subsequently lyse (burst) the bacterial cell. This precision targeting makes phage therapy exceptionally effective against multidrug-resistant organisms (MDROs) such as MRSA, Pseudomonas aeruginosa, and Acinetobacter baumannii. Historically utilized extensively in Eastern Europe, the therapy is now undergoing rigorous clinical evaluation and regulatory modernization in Western markets, notably the United States and Europe, facilitating its integration into mainstream clinical practice. Regulatory pathways, including compassionate use protocols and streamlined approval processes for personalized phage cocktails, are critical factors supporting market expansion.
Major applications of bacteriophage therapy include topical treatments for skin and soft tissue infections, oral delivery for gastrointestinal tract infections, intravenous delivery for systemic infections, and targeted use in cystic fibrosis patients suffering from chronic lung infections. The key driving factors are the urgent public health need to counter AMR, increased funding for alternative antimicrobial research by governmental and private entities, advancements in phage engineering (synthetic biology), and growing clinical evidence demonstrating efficacy and safety in human trials. Furthermore, the rising awareness among clinicians regarding the limitations of the current antibiotic pipeline is fueling demand for innovative therapeutic solutions like phages.
Bacteriophage Therapy Market Executive Summary
The Bacteriophage Therapy Market is poised for substantial growth, driven primarily by the urgent global requirement for solutions against multidrug-resistant (MDR) bacterial infections. Business trends indicate a shift from generalized research towards specialized product development, focusing on standardized, scalable phage cocktails rather than purely personalized treatments. Key pharmaceutical and biotechnology companies are increasingly engaging in strategic partnerships and collaborations with academic institutions and specialized phage centers (e.g., Phage Directory, Eliava Institute) to accelerate clinical trials and navigate complex regulatory landscapes. Investment flows are robust, concentrating particularly on developing manufacturing processes that meet stringent Good Manufacturing Practice (GMP) standards, a necessity for broad market acceptance.
Regional trends highlight North America and Europe as the primary revenue generators, characterized by high healthcare spending, advanced infrastructure for clinical research, and a clear regulatory push (e.g., FDA’s efforts to expedite novel antimicrobial approval). However, the Asia Pacific (APAC) region, including countries like South Korea, China, and India, is emerging as a critical growth hub due to high infection burdens, large patient populations, and lower relative costs for clinical trials. The historical precedent of phage use in Eastern European countries continues to influence adoption, providing a foundation of long-term safety data, though modern GMP standards are now mandatory across all geographies for commercial success.
Segment trends reveal that the human applications segment, specifically targeting chronic infections (such as diabetic foot ulcers and prosthetic joint infections) and acute systemic infections, dominates the market share. Within product type, standardized phage cocktails are expected to capture the highest growth rate due to their commercial scalability and ability to target multiple bacterial strains simultaneously, offering a balance between specificity and broad utility. Furthermore, diagnostic segments—specifically those identifying appropriate phage candidates quickly—are receiving focused R&D investment, underscoring the shift toward integrated diagnostic-therapeutic solutions (theranostics) essential for successful personalized treatment protocols.
AI Impact Analysis on Bacteriophage Therapy Market
User inquiries frequently center on how Artificial Intelligence (AI) can overcome the primary bottleneck in phage therapy: the precise and rapid identification, selection, and optimization of effective phages against specific bacterial pathogens, especially in personalized treatment scenarios. Users are concerned about the scalability of phage production and the complexity of predicting phage-bacteria interactions, asking whether machine learning models can accurately screen large libraries of phages and predict therapeutic efficacy and host range without extensive bench validation. The key themes revolve around AI's ability to revolutionize phage bioinformatics, accelerate discovery timelines, ensure safety profiles by detecting potential toxins, and optimize complex phage cocktail compositions, which require simultaneous effectiveness against multiple strains while minimizing resistance development.
AI’s influence is profound, transforming phage therapy from a labor-intensive, often bespoke process into a data-driven, scalable therapeutic modality. AI algorithms excel at analyzing vast genomic, proteomic, and clinical datasets generated from phage-bacteria interaction studies. This capability significantly shortens the lead time required to match a patient's resistant bacterial strain with the optimal lytic phage or cocktail. By employing neural networks and predictive modeling, researchers can rapidly identify favorable phage characteristics, such as broad lytic activity, stability, and absence of lysogenic genes or harmful virulence factors, thereby streamlining the path from discovery to clinical application and enhancing the safety margin of commercialized products.
- AI-driven personalized phage selection: Machine learning models predict the most effective phage candidates based on bacterial genomic sequences and resistance patterns.
- Accelerated Phage Discovery: Utilization of deep learning for rapid screening and annotation of novel phages from environmental and cultured samples.
- Optimization of Phage Cocktails: Algorithms determine the ideal ratio and combination of multiple phages to prevent bacterial resistance evolution and maximize killing efficacy.
- Enhanced Manufacturing Scalability: AI optimizes fermentation and purification parameters, ensuring high yield and quality control (GMP compliance).
- Safety and Virulence Prediction: Automated analysis of phage genomes to detect integration sites, lysogenic traits, or potential toxins, ensuring clinical safety.
- Clinical Trial Design Efficiency: AI analyzes patient response data to stratify patients, predict treatment outcomes, and refine dosing strategies in real-time.
DRO & Impact Forces Of Bacteriophage Therapy Market
The Bacteriophage Therapy Market is shaped by powerful driving forces centered on the global antimicrobial resistance crisis and significant scientific opportunities, while being constrained by complex regulatory requirements and manufacturing hurdles. The primary driver remains the alarming failure rate of traditional antibiotics against critical priority pathogens, creating a desperate clinical need that phages are uniquely positioned to address. Opportunities arise from technological advancements, particularly in synthetic biology and genetic engineering, which allow for the modification of phages to enhance stability, broaden host range, or prevent premature resistance. These elements collectively exert a strong impact force pushing the market toward commercial maturity.
Restraints, however, pose significant challenges to widespread adoption. Regulatory uncertainty remains a major impediment; although pathways like compassionate use exist, achieving standardized, large-scale regulatory approval (like a traditional New Drug Application) is complex due to the highly specific, often variable nature of phage components. Manufacturing and quality control (QC) also present restraints; ensuring consistent potency, purity, and stability across personalized or small-batch phage cocktails requires establishing costly and specialized Good Manufacturing Practice (GMP) facilities. Furthermore, market penetration is hindered by the limited specialized clinical knowledge and infrastructure required for effective diagnosis, phage selection, and therapeutic delivery in mainstream hospitals.
The impact forces are predominantly concentrated on the healthcare system’s resilience against infection. The high mortality rates and increased healthcare costs associated with MDR infections provide an undeniable financial and ethical imperative for adopting effective alternatives like phages. The societal impact of preserving the efficacy of existing antibiotics by utilizing phages as a synergistic or replacement therapy further strengthens the market dynamics. Strategic collaborations between biotech companies, governmental bodies (e.g., BARDA), and non-profit organizations are amplifying the positive impact forces, accelerating research translation and addressing key regulatory gaps necessary for commercial success.
Segmentation Analysis
The Bacteriophage Therapy Market is comprehensively segmented based on its source material, application area, target delivery mechanism, and the type of product offered. This granular segmentation is crucial for understanding specific market dynamics, investment priorities, and areas of high clinical utility. The core segmentation reflects the dual nature of phage products: highly tailored, personalized solutions (often under compassionate use) versus standardized, commercially scalable cocktails designed for common, recurrent infections. The Application segment, particularly chronic bacterial infections and wound care, dominates due to the large, identifiable patient base suffering from persistent infections untreatable by standard drug regimens.
By Product Type, the market heavily favors Bacteriophage Cocktails. Although monotherapy (using a single phage) is utilized, cocktails offer a critical strategic advantage: they mitigate the rapid development of bacterial resistance against a single phage type and ensure efficacy against a wider spectrum of strains within a target species. This reliability is vital for commercial viability and regulatory acceptance. Furthermore, the segmentation by Target Area—human versus veterinary applications—shows that while human therapy is the primary revenue driver due to high clinical need and payment capacity, veterinary and agricultural applications represent high-growth potential, particularly in addressing antibiotic use in livestock.
The increasing refinement in delivery mechanisms, categorized into topical, oral, and systemic (intravenous/intramuscular), also dictates market direction. Topical application is currently the most commercially mature and readily accepted route due to its localized action and minimal systemic risk, making it ideal for skin and soft tissue infections. However, systemic delivery for severe, invasive infections like sepsis and osteomyelitis is a high-value, high-risk segment attracting intense R&D investment, representing the future frontier of critical care phage therapy.
- By Product Type:
- Bacteriophage Cocktails
- Monophage Products
- By Target Bacteria:
- Staphylococcus spp. (e.g., MRSA)
- Pseudomonas aeruginosa
- Escherichia coli (E. coli)
- Acinetobacter baumannii
- Klebsiella pneumoniae
- Others (e.g., Clostridium difficile, Salmonella spp.)
- By Application:
- Chronic Bacterial Infections (e.g., Diabetic Foot Ulcers, Cystic Fibrosis related lung infections)
- Skin and Soft Tissue Infections (SSTIs)
- Gastrointestinal Infections
- Respiratory Infections
- Systemic Infections (e.g., Sepsis, Bacteremia)
- Veterinary and Agriculture
- By Route of Administration:
- Topical
- Oral
- Systemic (Intravenous/Intramuscular/Intraperitoneal)
- Inhalation
Value Chain Analysis For Bacteriophage Therapy Market
The value chain for the Bacteriophage Therapy Market begins with rigorous upstream activities focused on discovery and characterization, progresses through highly specialized manufacturing, and concludes with downstream activities involving clinical application and distribution. Upstream analysis involves environmental sampling (e.g., wastewater, soil) to isolate novel phages, followed by genomic sequencing and extensive characterization to confirm lytic activity, host range, and absence of undesirable genes (virulence or lysogeny). This stage is heavily reliant on academic collaboration, specialized biobanks, and bioinformatics tools, defining the quality and potential efficacy of the final therapeutic product.
Midstream activities—manufacturing and formulation—represent the critical bottleneck and value addition stage. Phages must be produced at scale under stringent GMP conditions, which includes controlled bacterial fermentation, purification (often involving filtration or centrifugation to remove endotoxins and bacterial debris), and precise formulation into stable, injectable, oral, or topical products. Unlike chemical drugs, the living nature of the product requires highly specialized quality control tests to measure titer (concentration) and stability. This necessity drives up manufacturing complexity and cost, but also creates a significant barrier to entry, concentrating market power among companies capable of meeting these high standards.
Downstream analysis covers distribution channels and end-user delivery. Distribution is highly regulated, often necessitating cold chain management to maintain product viability. Direct distribution is common in compassionate use scenarios, flowing directly from specialized labs or compounding pharmacies to treating hospitals or physicians. However, for commercialized, standardized products, the channel transitions to indirect distribution through specialized pharmaceutical distributors focused on novel biopharmaceuticals. The end-users—hospitals, specialized infection control centers, and clinics—require significant educational support, making the sales channel deeply reliant on specialized medical liaison teams and partnerships with infectious disease specialists to drive adoption and ensure correct therapeutic use.
Bacteriophage Therapy Market Potential Customers
The primary and most critical segment of potential customers for the Bacteriophage Therapy Market comprises patients suffering from antibiotic-refractory bacterial infections, particularly those involving critical priority pathogens categorized by the WHO and CDC. This includes individuals with chronic, biofilm-associated infections such as prosthetic joint infections, diabetic foot ulcers that lead to amputation, and severe respiratory infections in immunocompromised or cystic fibrosis patients. These patients represent the highest unmet medical need, where conventional therapeutic failure necessitates immediate and innovative intervention, driving demand for personalized and effective phage treatments.
Beyond individual patients, the institutional buyers form the second major customer base. These include large tertiary care hospitals, specialized infectious disease clinics, burn units, and military medical centers, which frequently encounter multidrug-resistant outbreaks and complex infections. These institutions are potential bulk purchasers of standardized phage cocktails for immediate use, especially if the products gain broad regulatory approval. Furthermore, governments and public health organizations (e.g., national healthcare services, defense agencies) are key customers, funding stockpiles of phages as a biodefense strategy and investing in national programs to combat AMR, recognizing phages as a critical strategic asset.
The third tier of customers includes veterinary practices and agricultural producers. As regulatory pressure increases to reduce prophylactic antibiotic use in livestock, phages offer a viable alternative for treating and preventing common bacterial infections in animals (e.g., poultry, swine, cattle). This B2B segment is driven by economic viability and consumer safety demands. Finally, Contract Manufacturing Organizations (CMOs) and Contract Research Organizations (CROs) specializing in advanced biologics represent customers for highly specific, research-grade phages and related services, particularly those supporting early-stage biotech companies or large pharma exploring the phage pipeline.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 75.0 million |
| Market Forecast in 2033 | USD 170.0 million |
| Growth Rate | 12.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Adaptive Phage Therapeutics, Phage International, Inc., Phage Therapy Center, Phagomed, Eliava Bio Preparations, Intralytix, Inc., Locus Biosciences, Inc., Fixed-Phage Ltd., Technophage S.A., Micreos B.V., PhagePro, Inc., Armata Pharmaceuticals, Inc., Enbiotix, Inc., Wockhardt Ltd., AmpliPhi Biosciences Corporation (now Armata Pharmaceuticals), Phage UK, Phage Diagnostics, BioNTech. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Bacteriophage Therapy Market Key Technology Landscape
The technological evolution within the Bacteriophage Therapy Market is defined by three intersecting domains: phage isolation and characterization, synthetic biology for engineering, and sophisticated delivery systems. Traditional technology centers around manual plaque assays and electron microscopy for initial isolation, but modern platforms integrate high-throughput sequencing (NGS) and advanced bioinformatics tools (e.g., Phage Annotation Pipelines) for rapid genomic analysis. This shift from labor-intensive manual screening to data-driven identification is essential for creating large, well-characterized phage libraries, which forms the technological foundation for commercial viability and precise therapeutic matching in personalized medicine settings.
Synthetic biology and genetic engineering represent the most transformative technological advancements. Researchers are utilizing CRISPR-Cas systems and other gene editing tools to modify natural phages. This engineering addresses historical limitations by removing unwanted genes (e.g., genes promoting lysogeny or encoding toxins), broadening the phage’s lytic host range, or enhancing stability and target delivery specificity. The concept of "designer phages" moves beyond natural selection, promising products with predictable performance characteristics necessary for regulatory approval. Furthermore, the development of stable, long-lasting formulation technologies, such as microencapsulation or lyophilization, is crucial for standardizing product shelf life and global distribution under varying environmental conditions.
Advanced diagnostic technologies, often categorized as companion diagnostics, are integral to the therapeutic landscape. Rapid bacterial identification and antibiogram testing combined with high-speed phage sensitivity testing (phagogram) using automation and microfluidics are critical for reducing the treatment initiation timeline. Furthermore, phage manufacturing utilizes specialized bioreactors and purification techniques designed for biologics, distinct from traditional small molecule drug production. Technologies like tangential flow filtration (TFF) and chromatography are essential for removing trace endotoxins and host debris, ensuring the high purity required for systemic administration and clinical safety standards.
Regional Highlights
- North America: North America, particularly the United States, holds the largest market share and is expected to maintain its leadership through the forecast period. This dominance is attributed to substantial government and private sector funding directed towards AMR research, a highly developed healthcare infrastructure capable of supporting complex clinical trials, and the presence of numerous specialized biotech startups (e.g., Adaptive Phage Therapeutics, Locus Biosciences). The U.S. FDA’s increasing willingness to facilitate investigational use through Expanded Access programs (compassionate use) has accelerated clinical experience. However, regulatory harmonization and large-scale, standardized product approval remain high-priority hurdles for full commercialization.
- Europe: Europe represents a mature but complex market, historically influenced by Eastern European countries (e.g., Georgia, Poland) where phage therapy has been in continuous use. Western European countries are now rapidly catching up, driven by the strong public health focus on AMR and robust regulatory initiatives (e.g., European Medicines Agency efforts). Countries like Belgium and France have established national frameworks for the regulated use of personalized phages. Investment is particularly high in Germany and the UK, focusing on developing GMP-compliant manufacturing facilities and establishing clinical guidelines for integrating phages into standard care protocols for conditions like bone and joint infections.
- Asia Pacific (APAC): The APAC region is projected to register the fastest growth rate. This rapid expansion is fueled by high population density, increasing prevalence of MDR infections (especially in countries like India and China), and growing government initiatives to invest in biopharma innovation. South Korea and China are emerging as key R&D hubs, leveraging lower operational costs and large patient cohorts for clinical trials. The market growth here is driven by both clinical need and a relatively pragmatic approach to regulatory pathways for novel antimicrobials, positioning APAC as a critical region for future manufacturing and consumption of commercial phage products.
- Latin America: The Latin American market for bacteriophage therapy is nascent but demonstrates high potential, particularly in countries like Brazil and Mexico, which face high burdens of hospital-acquired infections (HAIs) and limited access to the latest generation of antibiotics. Market development is currently concentrated on localized research efforts and academic collaborations with global phage centers. Economic instability and differing regulatory maturities across countries pose constraints, but the unmet need for effective antimicrobials ensures a steady, albeit slow, growth trajectory, often driven by initial implementation through governmental public health programs targeting specific endemic pathogens.
- Middle East and Africa (MEA): The MEA region presents a market characterized by high infectious disease prevalence and significant variation in healthcare standards. The Middle East, particularly UAE and Saudi Arabia, shows high potential due to significant healthcare spending and investments in advanced medical technologies, potentially acting as early adopters of innovative therapies for treating antibiotic-resistant wound infections and sepsis. Conversely, Africa's market development is currently bottlenecked by infrastructure and affordability, though the acute need for cost-effective antimicrobial solutions positions phages as a promising long-term strategic option, especially in veterinary and local human health contexts.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Bacteriophage Therapy Market.- Adaptive Phage Therapeutics (APT)
- Intralytix, Inc.
- Locus Biosciences, Inc.
- Armata Pharmaceuticals, Inc.
- Eliava Bio Preparations (EBP)
- Phage International, Inc.
- Micreos B.V. (now focused on endolysins and targeted antimicrobials)
- Fixed-Phage Ltd.
- Technophage S.A.
- Phagomed Biopharma GmbH
- PhagePro, Inc.
- BioNTech (Through partnerships and pipeline expansion)
- Wockhardt Ltd.
- iNtRON Biotechnology, Inc.
- Ampliphi Biosciences (acquired by Armata)
- GangaGen Inc.
- Phage Therapy Center
- Enbiotix, Inc.
- Cytophage Technologies Inc.
- Pylum Biosciences
Frequently Asked Questions
Analyze common user questions about the Bacteriophage Therapy market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary factor driving the growth of the Bacteriophage Therapy Market?
The foremost driver is the global crisis of Antimicrobial Resistance (AMR). As conventional antibiotics fail against multidrug-resistant pathogens, bacteriophages offer a highly specific and effective alternative, creating an urgent clinical and economic need for novel therapeutic agents.
What are the major challenges facing the commercialization of phage therapy?
Major challenges include navigating complex and often non-standardized regulatory pathways (especially for personalized phage cocktails), establishing scalable manufacturing processes compliant with Good Manufacturing Practice (GMP), and overcoming the lack of widespread clinical familiarity among healthcare professionals.
How does AI contribute to the development of bacteriophage products?
AI significantly accelerates phage discovery, characterization, and optimization. Machine learning algorithms analyze genomic data to rapidly identify effective lytic phages, predict host range, ensure safety by screening for virulence genes, and optimize the composition of complex phage cocktails.
Which application segment holds the largest market share in bacteriophage therapy?
The segment targeting chronic bacterial infections, particularly Skin and Soft Tissue Infections (SSTIs) and infections related to orthopedic implants or diabetic foot ulcers, holds the largest market share due to the persistent nature of these infections and high rates of antibiotic treatment failure.
Why is North America considered the leading region in the Bacteriophage Therapy Market?
North America leads due to high R&D investment, robust biopharmaceutical infrastructure, high prevalence of MDR infections requiring advanced treatment, and supportive regulatory mechanisms such as the FDA's Expanded Access pathway, which facilitates the clinical use of investigational phage therapies.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager