Codeine Phosphate Market Size, By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 439531 | Date : Jan, 2026 | Pages : 245 | Region : Global | Publisher : MRU
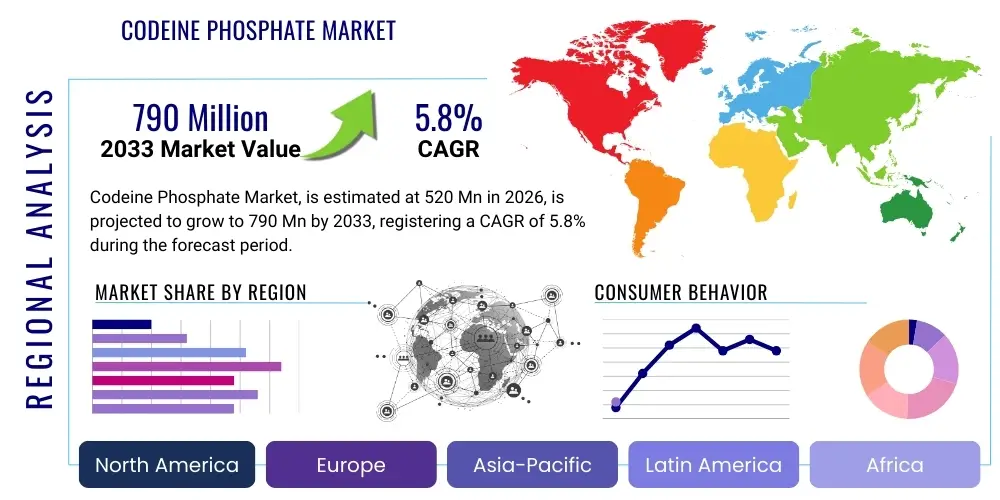
Codeine Phosphate Market Size
The Codeine Phosphate Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 5.8% between 2026 and 2033. The market is estimated at USD 520 Million in 2026 and is projected to reach USD 790 Million by the end of the forecast period in 2033.
Codeine Phosphate Market introduction
The Codeine Phosphate market encompasses the global production, distribution, and consumption of codeine phosphate, a widely recognized opioid analgesic and antitussive. Derived from opium, codeine phosphate is a semi-synthetic opioid primarily used for the relief of mild to moderate pain and for the symptomatic treatment of cough. Its efficacy in modulating pain signals in the central nervous system and suppressing the cough reflex has established its significant role in clinical practice across various medical disciplines. Major applications of codeine phosphate include its use in post-operative pain management, chronic pain conditions where non-opioid alternatives are insufficient, and in cough suppressants, often in combination with other active pharmaceutical ingredients to enhance therapeutic outcomes. The benefits of codeine phosphate lie in its well-understood pharmacokinetic profile, relatively lower abuse potential compared to stronger opioids, and its cost-effectiveness, particularly in generic formulations. The market is propelled by several key driving factors, including the rising global prevalence of chronic pain conditions, an increasing incidence of respiratory illnesses such as bronchitis and pneumonia that necessitate cough suppression, and the demographic shift towards an aging global population which is more susceptible to both pain and respiratory ailments. Furthermore, advancements in healthcare infrastructure in emerging economies are expanding access to essential medicines, thereby contributing to market growth. However, the market also navigates complex regulatory landscapes and public health concerns related to opioid misuse, influencing its trajectory.
Codeine Phosphate Market Executive Summary
The Codeine Phosphate market's executive summary reveals a complex interplay of evolving business dynamics, significant regional shifts, and diverse segmental trends. In terms of business trends, pharmaceutical companies are increasingly focusing on generic formulations to capture market share, alongside strategic partnerships and collaborations aimed at optimizing supply chain resilience and expanding geographical reach. There is also a discernible trend towards research and development into abuse-deterrent formulations, driven by global efforts to mitigate opioid misuse, which, while challenging, also presents opportunities for innovation within the market. Regulatory scrutiny remains a paramount business consideration, necessitating stringent compliance measures and influencing product development and market entry strategies. Regionally, North America continues to represent a substantial market share, primarily due to well-established healthcare systems and high diagnostic rates for pain and respiratory conditions, although it also faces the most stringent regulations and public health challenges related to opioid prescriptions. Europe maintains a steady demand, with market dynamics varying across countries based on national healthcare policies and prescription guidelines. The Asia Pacific region is poised for significant growth, fueled by its large patient population, improving healthcare access, and increasing disposable incomes, which are collectively driving demand for effective pain and cough management solutions. Latin America, the Middle East, and Africa are emerging as promising markets, characterized by developing healthcare infrastructures and a growing awareness of available treatments. Segment-wise, the pain management application segment holds the largest share due to the widespread prevalence of various pain conditions, followed by cough suppression. Formulation trends indicate a strong demand for oral dosage forms (tablets, capsules, syrups) due to their convenience, while the end-user landscape sees hospitals and retail pharmacies as primary distribution channels, with online pharmacies gaining traction. The market's overall trajectory is one of cautious expansion, balancing therapeutic need with public health imperatives.
AI Impact Analysis on Codeine Phosphate Market
Common user questions regarding the impact of AI on the Codeine Phosphate Market often revolve around its potential to revolutionize drug discovery, enhance personalized medicine, optimize supply chain logistics, predict adverse drug reactions, and assist in regulatory compliance. Users are keen to understand how AI can streamline the development of new codeine-based therapies or safer alternatives, improve patient outcomes through data-driven dosing, ensure consistent availability of the drug, and mitigate risks associated with its use. Concerns frequently raised include the ethical implications of AI in healthcare, data privacy, the potential for algorithmic bias, and the readiness of regulatory frameworks to adapt to AI-driven innovations. There is a strong expectation that AI could profoundly transform various facets of the market, from initial research and development phases to post-market surveillance and patient management. The key themes that emerge are efficiency, safety, personalization, and operational optimization, all underpinned by the critical need for responsible AI deployment.
- AI can significantly accelerate the drug discovery process for novel opioid analgesics or safer codeine derivatives by rapidly analyzing vast datasets of chemical compounds, predicting their efficacy, and identifying potential lead candidates.
- Predictive analytics powered by AI can optimize the manufacturing processes for codeine phosphate, improving yield, reducing waste, and ensuring consistent quality, thereby enhancing overall operational efficiency.
- AI-driven supply chain management tools can forecast demand fluctuations more accurately, identify potential disruptions, and optimize inventory levels, ensuring a stable and reliable supply of codeine phosphate to patients globally.
- Personalized medicine, enabled by AI, can help clinicians determine optimal codeine phosphate dosages for individual patients based on genetic factors, comorbidities, and real-time physiological data, potentially reducing adverse effects and improving therapeutic efficacy.
- Advanced AI algorithms can monitor and analyze real-time patient data to detect patterns of misuse, abuse, or adverse drug reactions associated with codeine phosphate, thereby enhancing pharmacovigilance and informing public health interventions.
- AI can assist in navigating complex regulatory landscapes by automating the analysis of regulatory guidelines, ensuring compliance in manufacturing, marketing, and distribution, and facilitating faster approval processes for new formulations.
- Through data analysis, AI can help identify patient populations most likely to benefit from codeine phosphate, improving treatment targeting and potentially reducing unnecessary prescriptions.
DRO & Impact Forces Of Codeine Phosphate Market
The Codeine Phosphate market is significantly shaped by a confluence of drivers, restraints, opportunities, and pervasive impact forces that dictate its current state and future trajectory. Key drivers include the escalating global prevalence of chronic pain conditions, which continuously fuels demand for effective pain management solutions, alongside the rising incidence of respiratory diseases such as bronchitis, pneumonia, and severe coughs requiring symptomatic relief. An aging global population, inherently more susceptible to these conditions, further contributes to market expansion. The cost-effectiveness and widespread availability of generic codeine phosphate formulations also make it an accessible option in many healthcare systems, sustaining its demand. However, the market faces considerable restraints, primarily stemming from stringent regulatory controls implemented by health authorities worldwide to combat the pervasive opioid crisis. Concerns regarding the potential for abuse, addiction, and diversion of opioid medications, including codeine, lead to tighter prescription guidelines, restricted access, and enhanced monitoring. The increasing development and adoption of non-opioid pain relievers and alternative cough suppressants also pose a competitive threat, potentially eroding codeine phosphate's market share. Public health campaigns aimed at reducing opioid dependency and promoting safer pain management practices further constrain market growth. Despite these challenges, opportunities abound in emerging markets where healthcare infrastructure is rapidly improving and access to essential medicines is expanding. Research and development into abuse-deterrent formulations, combination therapies with synergistic effects, and the exploration of novel drug delivery systems represent promising avenues for market innovation and growth. Improved pain assessment tools and diagnostics also offer opportunities for more judicious and effective prescribing. The overarching impact forces influencing this market include the global regulatory landscape, which dictates manufacturing standards, prescription practices, and market access; public health initiatives and societal perceptions regarding opioid use; advancements in medical science and pharmaceutical technology; and the evolving competitive environment with both traditional and novel therapeutic options. These forces collectively create a dynamic and highly regulated market where therapeutic utility must be carefully balanced with public safety.
Segmentation Analysis
The Codeine Phosphate market is comprehensively analyzed through various segmentation categories, offering a granular view of its diverse landscape and enabling a deeper understanding of market dynamics, consumer preferences, and growth opportunities. These segmentations allow for targeted strategic planning and resource allocation by identifying key areas of demand and supply within the global market. The primary segmentation approaches typically consider the product type, its major applications, the different formulations available, and the end-user demographics, alongside crucial geographical breakdowns.
- By Product Type:
- Codeine Phosphate Hemihydrate
- Codeine Phosphate Anhydrous
- By Application:
- Pain Management (mild-to-moderate acute and chronic pain)
- Cough Suppression (dry cough, persistent cough associated with respiratory infections)
- Diarrhea (symptomatic relief of acute non-specific diarrhea)
- Other Applications (e.g., palliative care, specific veterinary uses)
- By Formulation:
- Tablets (immediate-release, extended-release)
- Capsules
- Syrups/Oral Solutions
- Injections (for severe cases or hospital settings)
- Suppositories
- By End-User:
- Hospitals (in-patient and out-patient pharmacies)
- Clinics (general practitioners, specialized clinics)
- Retail Pharmacies (community pharmacies, drug stores)
- Online Pharmacies/E-commerce
- Ambulatory Surgical Centers
- By Region:
- North America (U.S., Canada, Mexico)
- Europe (Germany, UK, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, India, Japan, South Korea, Australia, Rest of Asia Pacific)
- Latin America (Brazil, Argentina, Rest of Latin America)
- Middle East & Africa (UAE, Saudi Arabia, South Africa, Rest of MEA)
Value Chain Analysis For Codeine Phosphate Market
The value chain for the Codeine Phosphate market is a complex network of interconnected activities, beginning from the sourcing of raw materials to the final delivery to the end-user, illustrating the sequential stages that add value to the product. The upstream segment primarily involves the cultivation and processing of opium poppies, from which codeine is naturally derived, or the synthesis of codeine from other opioid precursors, followed by its conversion into codeine phosphate API (Active Pharmaceutical Ingredient) by specialized chemical and pharmaceutical manufacturers. This stage is crucial for ensuring the quality, purity, and consistent supply of the base compound. The midstream activities encompass the formulation of codeine phosphate into various dosage forms—such as tablets, capsules, syrups, or injections—by pharmaceutical companies, along with packaging, labeling, and quality control processes. This stage involves complex manufacturing operations, adherence to Good Manufacturing Practices (GMP), and rigorous testing to ensure product safety and efficacy. The downstream segment focuses on the distribution and commercialization of the finished pharmaceutical products. This includes a robust network of wholesalers, distributors, and logistics providers who transport the products to various points of sale and consumption. Distribution channels are typically categorized into direct and indirect channels. Direct distribution involves pharmaceutical companies supplying directly to large hospital networks, government health agencies, or major pharmacy chains. Indirect distribution, which is more common, involves sales through independent wholesalers, regional distributors, and third-party logistics (3PL) providers, who then supply to retail pharmacies, smaller clinics, and online pharmacies. The intricate interplay between these stages, coupled with stringent regulatory oversight at each step, defines the efficiency and integrity of the Codeine Phosphate market's value chain, ensuring the product reaches patients safely and effectively while managing costs and compliance.
Codeine Phosphate Market Potential Customers
The potential customers for codeine phosphate are diverse, encompassing a wide spectrum of end-users and buyers within the healthcare ecosystem, all driven by the need for effective pain management and cough suppression. Foremost among these are individual patients suffering from mild to moderate acute pain, such as post-operative pain, dental pain, musculoskeletal injuries, or headaches, as well as those managing chronic pain conditions like osteoarthritis or neuropathic pain where codeine offers a suitable therapeutic option. Patients experiencing persistent or severe coughs associated with various respiratory infections, bronchitis, or other pulmonary conditions also represent a significant end-user segment, relying on codeine's antitussive properties for symptomatic relief. Beyond individual patients, the institutional buyers form another critical customer group. Hospitals, including their inpatient and outpatient pharmacies, are major purchasers for use in post-operative care, emergency departments, and general medical wards. Clinics, encompassing general practitioners, specialized pain clinics, and pulmonology clinics, regularly prescribe and dispense codeine phosphate, making them key customers. Retail pharmacies, whether independent community pharmacies or large chain drugstores, serve as primary points of access for patients with prescriptions, acting as vital intermediaries in the supply chain. Furthermore, online pharmacies are emerging as increasingly important channels, catering to a growing consumer preference for convenience and home delivery. Healthcare providers themselves, including physicians, nurses, and pharmacists, are indirectly customers as they influence prescription patterns and product choices. The growing market for palliative care also identifies codeine phosphate as a relevant medication for managing discomfort and cough in patients with terminal illnesses. Lastly, veterinary practices can also be considered niche potential customers for specific animal pain relief or cough suppression applications, although this segment is comparatively smaller.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 520 Million |
| Market Forecast in 2033 | USD 790 Million |
| Growth Rate | 5.8% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Pfizer Inc., Johnson & Johnson, Sanofi S.A., GlaxoSmithKline plc, Novartis AG, AstraZeneca PLC, Merck & Co. Inc., Teva Pharmaceutical Industries Ltd., Mallinckrodt Pharmaceuticals, Hikma Pharmaceuticals PLC, Viatris Inc. (formerly Mylan N.V. and Upjohn), Lupin Pharmaceuticals Inc., Sun Pharmaceutical Industries Ltd., Cipla Ltd., Dr. Reddy's Laboratories Ltd., Purdue Pharma L.P. (historical context), Endo Pharmaceuticals Inc., Amneal Pharmaceuticals Inc., Indivior PLC, Grunenthal GmbH |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Codeine Phosphate Market Key Technology Landscape
The Codeine Phosphate market's key technology landscape is characterized by a blend of established pharmaceutical manufacturing processes, advanced analytical techniques, and evolving drug delivery systems, all aimed at ensuring product quality, efficacy, and patient safety while navigating complex regulatory demands. At the core are the synthesis and purification technologies for the Active Pharmaceutical Ingredient (API), which involve sophisticated chemical processes to convert raw materials, typically opium alkaloids, into high-purity codeine phosphate. These technologies are critical for consistency and adherence to stringent pharmacopoeial standards. Pharmaceutical formulation technologies play a pivotal role, encompassing the development of various dosage forms such as immediate-release and extended-release tablets, capsules, oral solutions, and injectables. This includes advancements in excipient science, which optimizes drug dissolution, bioavailability, and stability, as well as coating technologies to enhance patient compliance and protect the active ingredient. Controlled-release technologies are particularly significant for managing pain effectively over longer durations, reducing dosing frequency, and potentially mitigating the rapid onset of effects that can contribute to abuse potential. Furthermore, analytical technologies are indispensable across the entire value chain. High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), Mass Spectrometry (MS), and Nuclear Magnetic Resonance (NMR) are routinely employed for quality control, impurity profiling, and ensuring the identity and purity of both the API and the finished product. These advanced analytical methods are crucial for regulatory compliance and safeguarding patient health. In terms of drug delivery systems, innovations may include abuse-deterrent formulations (ADFs), which aim to prevent or deter common methods of abuse such as crushing, snorting, or injecting, thereby making the product less attractive for non-medical use. While challenging for codeine, research in this area explores physical or chemical barriers, antagonist combinations, or prodrug approaches. Lastly, digital technologies, including process automation in manufacturing and data analytics for pharmacovigilance, are increasingly integrated to enhance efficiency, reduce human error, and improve post-market surveillance of codeine phosphate products.
Regional Highlights
- North America: The largest market share holder, driven by a high prevalence of chronic pain and respiratory conditions, sophisticated healthcare infrastructure, and high per capita healthcare spending. However, stringent regulatory frameworks and aggressive public health campaigns to address the opioid crisis significantly impact prescription volumes and market growth, leading to a focus on responsible prescribing and alternative pain management strategies. The U.S. remains the dominant country within this region, facing intense scrutiny over opioid distribution.
- Europe: A mature market characterized by a stable demand for codeine phosphate, with market dynamics influenced by diverse national healthcare policies, drug reimbursement schemes, and varying regulatory stances on opioid prescribing across countries like the UK, Germany, and France. There is a strong emphasis on balancing pain relief with addiction prevention, leading to controlled distribution channels and robust pharmacovigilance.
- Asia Pacific (APAC): Projected to be the fastest-growing region, fueled by its immense population base, improving healthcare access and infrastructure, rising disposable incomes, and an increasing awareness of available pain and cough treatments. Countries like China and India present significant growth opportunities due to their large patient pools and evolving regulatory environments that are gradually becoming more amenable to pharmaceutical market expansion.
- Latin America: An emerging market demonstrating steady growth, driven by expanding healthcare expenditure, increasing prevalence of chronic diseases, and a growing demand for effective and affordable pain and cough remedies. Market expansion is supported by improving economic conditions and greater access to modern medicines, although regulatory harmonization across diverse nations remains a factor.
- Middle East & Africa (MEA): A developing market with considerable untapped potential, characterized by improving healthcare facilities, increasing investments in the pharmaceutical sector, and a rising burden of chronic illnesses. Market growth is gradual, influenced by socioeconomic development, healthcare reform initiatives, and varying levels of regulatory oversight and cultural sensitivities regarding opioid use.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Codeine Phosphate Market.- Pfizer Inc.
- Johnson & Johnson
- Sanofi S.A.
- GlaxoSmithKline plc
- Novartis AG
- AstraZeneca PLC
- Merck & Co. Inc.
- Teva Pharmaceutical Industries Ltd.
- Mallinckrodt Pharmaceuticals
- Hikma Pharmaceuticals PLC
- Viatris Inc.
- Lupin Pharmaceuticals Inc.
- Sun Pharmaceutical Industries Ltd.
- Cipla Ltd.
- Dr. Reddy's Laboratories Ltd.
- Purdue Pharma L.P.
- Endo Pharmaceuticals Inc.
- Amneal Pharmaceuticals Inc.
- Indivior PLC
- Grunenthal GmbH
Frequently Asked Questions
Analyze common user questions about the Codeine Phosphate market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is codeine phosphate primarily used for?
Codeine phosphate is primarily used as an opioid analgesic to relieve mild to moderate pain and as an antitussive to suppress cough. It is effective in managing various types of pain, including post-operative and chronic conditions, and in alleviating persistent coughs associated with respiratory illnesses. Its dual action makes it a versatile medication in clinical practice.
What are the main drivers of growth in the Codeine Phosphate Market?
The key drivers of market growth include the increasing global prevalence of chronic pain conditions, the rising incidence of respiratory diseases requiring cough suppression, and the demographic trend of an aging population more susceptible to these ailments. Additionally, the availability of cost-effective generic formulations contributes significantly to market expansion and accessibility.
How do regulatory policies impact the Codeine Phosphate Market?
Regulatory policies have a substantial impact, often acting as a restraint due to stringent controls aimed at combating opioid misuse and addiction. Governments worldwide implement strict prescription guidelines, monitoring programs, and classification of codeine, which influences market access, product development, and the overall volume of prescriptions, necessitating careful compliance by manufacturers and healthcare providers.
What are the major challenges faced by the Codeine Phosphate Market?
Major challenges include the ongoing opioid crisis concerns leading to increased regulatory scrutiny, the potential for abuse and addiction, and the growing competition from non-opioid pain relievers and alternative cough suppressants. Public health campaigns promoting safer pain management and reducing opioid dependence also pose significant hurdles for market growth and perception.
Which geographical region holds the largest share in the Codeine Phosphate Market?
North America currently holds the largest market share for Codeine Phosphate. This dominance is attributed to advanced healthcare infrastructure and a high prevalence of pain and respiratory conditions. However, the region also faces the most rigorous regulations and public health challenges related to opioid use, prompting continuous reevaluation of prescribing practices.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager