Dermal Filler Cannula Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 432305 | Date : Dec, 2025 | Pages : 241 | Region : Global | Publisher : MRU
Dermal Filler Cannula Market Size
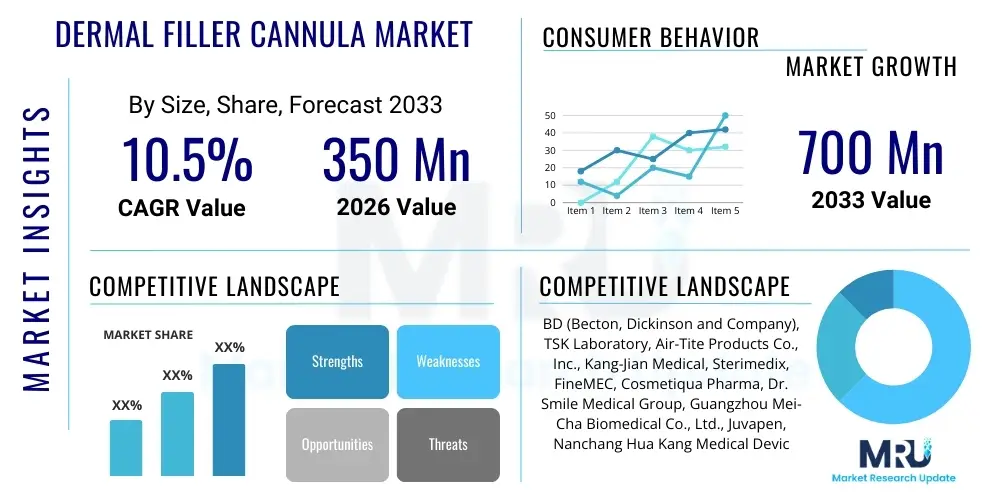
The Dermal Filler Cannula Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 10.5% between 2026 and 2033. The market is estimated at USD 350 Million in 2026 and is projected to reach USD 700 Million by the end of the forecast period in 2033.
Dermal Filler Cannula Market introduction
The Dermal Filler Cannula Market encompasses specialized medical devices designed for the precise and less traumatic delivery of injectable dermal fillers. Unlike traditional sharp needles, cannulas feature a blunt tip and a side aperture, allowing practitioners to navigate tissue planes, including those near delicate vascular structures and nerves, with significantly reduced risk of bruising, swelling, and vascular occlusion. This foundational benefit—enhanced safety coupled with superior patient comfort—is driving the rapid adoption of blunt-tip microcannulas across the aesthetic medicine landscape globally. The market growth is intricately linked to the escalating consumer demand for non-surgical cosmetic enhancements, particularly facial rejuvenation and volume restoration, positioning cannulas as an indispensable tool for advanced injection techniques.
Products within this sector are characterized by variations in length, gauge (diameter), and flexibility, enabling customization for different anatomical areas, such as the lips, cheeks, temples, and tear troughs. Major applications include facial sculpting, deep tissue volumization, fine line correction, and hand rejuvenation. The primary driving factors for market expansion include the substantial increase in the aging population globally, which seeks effective anti-aging solutions, and the ongoing shift from invasive surgical procedures to minimally invasive alternatives. Furthermore, continuous technological advancements focused on improving cannula tip design, surface coatings for smoother insertion, and ergonomic features are fueling professional preference and adoption rates in specialized dermatology and plastic surgery practices.
The market environment is highly competitive, characterized by stringent regulatory oversight, particularly regarding Class II medical devices in major jurisdictions like the FDA and EMA. Benefits derived from utilizing cannulas extend beyond patient safety, offering clinicians improved procedural efficiency, reduced material waste due to more precise placement, and higher overall patient satisfaction, which contributes directly to repeat business. These instruments are pivotal in executing advanced techniques such as fanning, cross-hatching, and tunneling, which are often required for creating natural-looking, homogeneous results, particularly in areas requiring large-volume product distribution or specific layering within the subdermal fat or subcutaneous tissue layers.
Dermal Filler Cannula Market Executive Summary
The Dermal Filler Cannula Market is experiencing robust acceleration, primarily propelled by favorable shifts in consumer preferences towards aesthetic treatments that minimize downtime and procedural risk. Key business trends indicate a strong focus on innovation related to needle gauge optimization and material sciences, specifically the integration of stainless steel alloys that balance flexibility with rigidity for optimal tissue handling. The market is witnessing consolidation among smaller specialized manufacturers and strategic acquisitions by large multinational medical device companies aiming to broaden their portfolio of aesthetic injectables and associated delivery systems. Furthermore, intense focus on standardized training protocols for practitioners in the use of microcannulas is becoming a critical success factor, influencing product loyalty and safe market penetration.
Regionally, North America maintains its dominance due to high disposable incomes, significant consumer awareness regarding aesthetic treatments, and the presence of leading key opinion leaders (KOLs) who advocate for cannula use in advanced procedures. However, the Asia Pacific region, particularly China and South Korea, is projected to register the highest Compound Annual Growth Rate (CAGR), driven by the burgeoning medical tourism sector, increasing accessibility of cosmetic procedures, and cultural acceptance of aesthetic enhancements. European markets remain mature but exhibit consistent growth, largely supported by strong regulatory frameworks that standardize product quality and ensure high levels of practitioner training, bolstering consumer trust in minimally invasive cosmetic interventions.
Segment trends reveal that the blunt-tip/microcannula segment dominates the market by product type, reflecting the industry's prioritization of safety and reduction of complications associated with traditional sharp needles, such as severe bruising and potential vascular trauma. In terms of application, facial augmentation, particularly lip and cheek volumization, remains the largest revenue contributor. The hospital and specialized dermatology clinic end-user segments are primary drivers of demand, although medical spas are rapidly expanding their market share. The overarching executive summary emphasizes that continuous safety improvements, coupled with effective professional training and rising global demand for subtle aesthetic results, solidify the Dermal Filler Cannula Market’s trajectory towards sustainable high-growth rates throughout the forecast period.
AI Impact Analysis on Dermal Filler Cannula Market
User queries regarding the impact of Artificial Intelligence (AI) on the Dermal Filler Cannula Market frequently center on themes such as procedural standardization, risk mitigation, and automated training. Users often question if AI algorithms can predict optimal injection depths or patterns, thereby guiding the cannula user in real-time. Concerns are also raised about whether AI-driven imaging systems could identify sub-surface vascular maps instantly, reducing the already minimized risk of vascular occlusion inherent with blunt cannulas. Furthermore, the role of AI in analyzing patient outcomes, identifying complication patterns related to cannula usage, and personalizing treatment plans based on demographic data and tissue density profiles represents a major area of public and professional interest, moving beyond basic product manufacturing and into the clinical application space.
AI's primary influence on the clinical application of dermal filler cannulas lies in enhanced procedural accuracy and diagnostic support. AI-powered imaging technologies, such as enhanced ultrasound or 3D facial mapping systems integrated with deep learning algorithms, can provide dynamic guidance to practitioners. These systems help map critical anatomical structures, estimate tissue viscosity, and calculate the optimal volume and placement trajectory for the filler material delivered via the cannula. This integration enhances the core benefit of the cannula—safety—by adding a layer of intelligent real-time confirmation, minimizing guesswork and maximizing the homogeneity of filler distribution. For example, AI can analyze thousands of successful procedure outcomes to suggest the most appropriate cannula gauge and length for a specific aesthetic goal (e.g., tear trough correction) in a given facial structure.
From a commercial and manufacturing standpoint, AI facilitates predictive maintenance of manufacturing equipment used for high-precision cannula production, ensuring consistent quality and reduced defect rates crucial for these specialized medical instruments. Moreover, AI models are increasingly utilized in market trend forecasting, helping manufacturers predict demand for specific cannula types (e.g., flexible vs. rigid) based on evolving regional aesthetic preferences and the adoption rate of new filler formulations. Ultimately, AI transforms the market by elevating the safety profile, automating complex decision-making processes during procedures, and creating highly personalized training simulators for novice cannula users, thereby accelerating the adoption of best practice injection techniques across the globe.
- AI-Enhanced Diagnostic Imaging: Real-time guidance systems leveraging AI to identify vascular pathways and nerve locations, significantly lowering the risk of complications during filler injection using cannulas.
- Automated Training & Simulation: Development of virtual reality (VR) and augmented reality (AR) simulators, powered by AI, offering standardized, high-fidelity training for new practitioners on optimal cannula manipulation and technique.
- Predictive Outcome Analysis: AI algorithms assess patient facial topography and recommend optimal injection points, volume distribution, and cannula specifications for personalized, natural aesthetic results.
- Quality Control Optimization: Utilization of machine vision and deep learning in manufacturing processes to ensure the consistency of tip bluntness, aperture size, and overall cannula integrity, reducing production variance.
- Supply Chain & Inventory Forecasting: AI models predict regional demand shifts for specific cannula gauges and lengths based on seasonal trends and demographic data, optimizing inventory management for suppliers and distributors.
DRO & Impact Forces Of Dermal Filler Cannula Market
The Dermal Filler Cannula Market is subjected to a powerful set of dynamic forces encompassing Drivers (D), Restraints (R), and Opportunities (O), which collectively shape its expansion trajectory and competitive landscape. The primary driver is the pervasive global preference for minimally invasive aesthetic procedures, driven by consumer demand for reduced downtime, less pain, and lower risk of complications compared to traditional surgery. This aligns perfectly with the core advantage of blunt-tip cannulas, which substantially reduce bruising and vascular injury. Simultaneously, the impact force of increasing standardization and globalization of aesthetic training protocols actively promotes the adoption of cannulas as the safer, standard technique for complex facial areas. However, growth is inherently restrained by the high cost associated with premium cannulas compared to conventional needles, coupled with the need for specialized, mandatory training required for safe and effective usage, which can deter some practitioners or clinic environments with limited resources.
Opportunities for market stakeholders primarily emerge from the rapid expansion of the application scope of dermal fillers beyond traditional facial regions to areas like the neck, décolletage, hands, and buttocks, demanding longer and specialized cannulas for deep tissue placement and body contouring. Further opportunities are fueled by material science advancements, including the development of coated or lubricated cannulas designed to minimize friction during tissue passage, enhancing procedural smoothness and patient comfort. The regulatory environment acts as a dual-edged force; while stringent regulation (a restraint) requires significant investment in clinical data and compliance, it also raises the barrier to entry, favoring established, high-quality manufacturers, reinforcing trust, and acting as an impact force driving product quality improvement.
The cumulative impact forces dictate a sustained growth environment, contingent upon manufacturers' ability to maintain technological superiority while addressing price sensitivity, particularly in emerging markets. The positive impact of media influence and social acceptance of aesthetic treatments significantly bolsters demand, transforming what were once considered luxury procedures into mainstream consumer services. The constant threat of malpractice lawsuits associated with filler complications, even when using cannulas, necessitates continuous innovation focused on safety features, which acts as a powerful, sustained impact force compelling practitioners toward safer tools, thus favoring the microcannula segment over sharp needles.
Segmentation Analysis
The Dermal Filler Cannula Market is structurally segmented based on crucial dimensions, including Product Type, Application, Material, and End-User. This segmentation provides a granular view of market dynamics, revealing that the primary differentiation driver remains the tip design and flexibility, directly correlating with procedural safety and outcome. The segmentation analysis underscores the trend of specialized product development, where manufacturers are increasingly focusing on tailoring cannulas—in terms of gauge and length—to specific anatomical regions and filler viscosities, optimizing both delivery precision and patient recovery time. Understanding these segments is vital for stakeholders to allocate resources effectively, target specific professional groups, and align product development strategies with clinical demands across various geographies.
The dominance of the Blunt Tip/Microcannula segment reflects a fundamental industry commitment to mitigating the risks associated with sharp needle injections, making this classification the high-growth powerhouse of the market. Similarly, the Application segment confirms that the vast majority of dermal filler procedures are centered around facial aesthetics, necessitating tools capable of navigating complex facial vasculature. Analyzing the End-User segmentation reveals a critical reliance on specialized medical settings, ensuring that procedures are conducted by trained professionals, thereby reinforcing quality and safety standards across the utilization spectrum. The complex interplay between these segments ultimately drives pricing strategies and dictates the volume of distribution channels utilized by major manufacturers.
- Product Type: Blunt Tip/Microcannula, Sharp Tip Cannula
- Application: Facial Augmentation (Cheeks, Lips, Tear Trough, Nasolabial Folds), Body Contouring (Hands, Neck, Decolletage, Others)
- Material: Stainless Steel, Others (Polymer-based, Specialized Alloys)
- End-User: Dermatology Clinics and Specialized Practices, Hospitals, Medical Spas and Beauty Centers
- Gauge Size: 18G-20G (Large Volume), 21G-25G (Medium Volume), 27G-30G (Fine Detail/Superficial)
Value Chain Analysis For Dermal Filler Cannula Market
The value chain for the Dermal Filler Cannula Market begins with the upstream sourcing of specialized medical-grade raw materials, primarily high-quality stainless steel alloys, which must meet stringent biocompatibility and mechanical strength standards. Key activities at this stage include precision material procurement, advanced sterilization processes, and the utilization of complex micro-machining technologies necessary to create the blunt, highly polished tip and the specific side aperture of the microcannulas. Manufacturers invest heavily in R&D to optimize surface treatments and flexibility profiles. The highly technical nature of blunt-tip production requires specialized tooling and rigorous quality control measures, differentiating this manufacturing segment from standard needle production. This upstream reliance on specialized engineering skills and materials significantly influences the final product cost and quality.
The central manufacturing phase involves assembly, cleaning, packaging, and regulatory compliance clearance, which is followed by the core distribution channel strategy. The distribution channels are bifurcated into direct and indirect routes. Direct sales often target large hospital groups, key opinion leader clinics, and national distributors that operate under exclusive contracts. Indirect channels rely on a network of regional medical supply distributors, aesthetic product wholesalers, and authorized resellers, particularly for penetrating fragmented markets or reaching smaller medical spas. The effectiveness of the supply chain is heavily dependent on maintaining cold chain or controlled environment logistics, ensuring product sterility and integrity until the point of use.
Downstream analysis focuses on the end-user segments, which include specialized dermatology clinics, plastic surgery centers, and certified medical spas. The critical component of the downstream value chain is professional training and education, often provided or sponsored by the manufacturer or distributor, which is essential for ensuring safe usage and maximizing product utility. The ultimate buyer (the patient) is influenced by the aesthetic practitioner’s choice, making the relationship between manufacturers and clinical professionals paramount. The value chain concludes with post-market surveillance and continuous feedback loops, which inform future product iterations and quality improvements, solidifying the continuous refinement necessary for medical devices in the aesthetic sector.
Dermal Filler Cannula Market Potential Customers
The primary customers and end-users of dermal filler cannulas are highly specialized medical professionals and institutions dedicated to aesthetic and reconstructive medicine. These include board-certified dermatologists, plastic surgeons, and specialized aesthetic physicians who routinely perform minimally invasive procedures such as facial volume restoration, wrinkle reduction, and tissue contouring. The decision to purchase specific cannula brands is often driven by factors such as procedural complexity, the type and viscosity of the dermal filler being used (e.g., Hyaluronic Acid, Calcium Hydroxylapatite), and the need for tools that offer the highest combination of safety and precision, ensuring minimal trauma to the patient.
Secondary but rapidly growing customer segments include high-end medical spas and non-hospital ambulatory surgical centers that possess the requisite licensing and staff training to administer dermal fillers. These facilities represent a significant volume consumer base, driven by accessibility and competitive pricing models, particularly in metropolitan areas. The purchasing decisions in these centers are often centralized and influenced by cost-efficiency, bulk purchasing discounts, and product reliability. Furthermore, specialized training institutions and academic centers that focus on aesthetic medicine are key customers, using cannulas for educational purposes and standardizing techniques for future practitioners.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 350 Million |
| Market Forecast in 2033 | USD 700 Million |
| Growth Rate | 10.5% ( Include CAGR Word with % Value ) |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | BD (Becton, Dickinson and Company), TSK Laboratory, Air-Tite Products Co., Inc., Kang-Jian Medical, Sterimedix, FineMEC, Cosmetiqua Pharma, Dr. Smile Medical Group, Guangzhou Mei-Cha Biomedical Co., Ltd., Juvapen, Nanchang Hua Kang Medical Device Co., Ltd., Biomatrix, HansBiomed, CosmoFrance, L'Essence, Embellash Medical, Dermalab International, NeedleTech Products Inc., Cequence, Micro Aesthetic Cannula. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Dermal Filler Cannula Market Key Technology Landscape
The Dermal Filler Cannula Market is defined by continuous technological refinement focused on enhancing precision, minimizing tissue trauma, and optimizing filler flow dynamics. The primary technological advancements revolve around specialized tip architecture. Modern cannulas utilize patented blunt-tip designs that minimize the risk of piercing vascular structures, achieved through sophisticated electro-polishing and grinding techniques that create a seamlessly smooth, rounded end. Further innovation is seen in the side aperture, which is engineered for controlled, even disbursement of highly viscous dermal fillers, often requiring computer numerical control (CNC) milling for precise placement relative to the tip curvature, ensuring optimal material delivery with minimal resistance during injection.
Material science and manufacturing processes also represent a core technological area. High-grade stainless steel (often surgical 304 or 316 variants) is the standard, but manufacturers are increasingly employing specialized coatings, such as silicone or proprietary polymer films, to reduce friction resistance as the cannula traverses multiple tissue layers. This lubricity technology significantly enhances the tactile feedback for the practitioner and reduces patient discomfort. Furthermore, advancements in hub design—the connection point between the cannula and the syringe—are critical, incorporating features like ergonomic grips and Luer lock standardization to ensure secure, leak-proof connections, which is paramount when injecting expensive filler material under high pressure.
A recent significant technological trend involves the integration of flexible yet kink-resistant tubing, particularly important for longer cannulas used in body contouring or large-area facial treatments. This balance of flexibility and structural integrity allows for broader sweeping motions within the subcutaneous plane without risk of breakage or deformation. Finally, the sterilization technology, typically gamma irradiation or ethylene oxide (EO), must be rigorously controlled to ensure absolute product safety without compromising the integrity of the specialized material coatings or the ultra-fine tip structures, reflecting an ongoing technological investment in validated terminal sterilization protocols compliant with global medical device standards.
Regional Highlights
- North America: This region maintains the largest market share, driven by high consumer spending on aesthetic procedures, deep penetration of aesthetic device technology, and robust regulatory structures (FDA) that facilitate the rapid adoption of new, safe products. The U.S. and Canada benefit from a large density of highly trained practitioners and a strong presence of global market leaders, making it a critical market for product launch and innovation validation.
- Europe: The European market is mature and characterized by high standardization, driven by the Medical Device Regulation (MDR) which mandates rigorous testing and conformity assessment, favoring established high-quality brands like those originating in Germany and France. Growth is steady, fueled by increasing consumer acceptance in Western European countries and expanding aesthetic markets in Eastern Europe, with a strong preference for cannulas due to the emphasis on patient safety within the public health consciousness.
- Asia Pacific (APAC): APAC is the fastest-growing market globally, propelled by rising disposable incomes, the burgeoning medical tourism sector (especially in South Korea and Thailand), and a cultural emphasis on aesthetic appearance. China and India represent significant potential due to vast population size and improving healthcare infrastructure. The rapid adoption rate is often associated with the influence of South Korean aesthetic trends and local manufacturing capabilities, leading to aggressive pricing and market competition.
- Latin America (LATAM): Growth in LATAM is moderate but consistent, largely driven by countries like Brazil and Mexico, which have large numbers of certified plastic surgeons and dermatologists, and a high cultural acceptance of cosmetic procedures. Market challenges include economic volatility and fragmented distribution channels, yet the high volume of aesthetic procedures performed maintains a steady demand for cost-effective, reliable cannula delivery systems.
- Middle East and Africa (MEA): This region is characterized by high growth in specific Gulf Cooperation Council (GCC) countries (UAE, Saudi Arabia) where high-net-worth individuals drive demand for premium, high-quality aesthetic services. The reliance on medical imports and the establishment of international clinic franchises are key factors. Market penetration is slower in Africa, but increasing investment in private healthcare facilities promises future growth opportunities for basic and intermediate cannula products.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Dermal Filler Cannula Market.- BD (Becton, Dickinson and Company)
- TSK Laboratory
- Air-Tite Products Co., Inc.
- Kang-Jian Medical
- Sterimedix
- FineMEC
- Cosmetiqua Pharma
- Dr. Smile Medical Group
- Guangzhou Mei-Cha Biomedical Co., Ltd.
- Juvapen
- Nanchang Hua Kang Medical Device Co., Ltd.
- Biomatrix
- HansBiomed
- CosmoFrance
- L'Essence
- Embellash Medical
- Dermalab International
- NeedleTech Products Inc.
- Cequence
- Micro Aesthetic Cannula
Frequently Asked Questions
Analyze common user questions about the Dermal Filler Cannula market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the key advantage of using a microcannula over a sharp needle for dermal fillers?
The primary advantage is enhanced patient safety and reduced procedural trauma. Microcannulas have a blunt tip and are highly flexible, allowing them to push blood vessels aside rather than pierce them, drastically reducing the risks of bruising, swelling, and severe complications like vascular occlusion.
Which geographical region exhibits the fastest growth rate in the Dermal Filler Cannula Market?
The Asia Pacific (APAC) region, driven by countries like South Korea and China, is projected to record the highest Compound Annual Growth Rate (CAGR) due to rapid aesthetic market expansion, increasing medical tourism, and growing consumer demand for advanced cosmetic treatments.
What are the most commonly targeted application areas for dermal filler cannulas?
Facial Augmentation is the dominant application segment. Specifically, cannulas are frequently used for precise and safe filler placement in sensitive areas such as the cheeks, lips, nasolabial folds, and the tear trough (under-eye area), optimizing results while minimizing discomfort.
How does technological innovation impact the current market landscape for cannulas?
Technological innovation primarily focuses on improving tip smoothness and side aperture engineering for optimized filler flow, along with specialized surface coatings (like silicone) to reduce friction. These advancements lead to superior precision, better patient comfort, and reduced procedural time, driving market preference for premium products.
What regulatory classification do dermal filler cannulas typically fall under in major markets?
In major jurisdictions like the United States and Europe, dermal filler cannulas are generally classified as Class II medical devices. This classification requires manufacturers to meet specific performance standards, conduct clinical data analysis, and adhere to stringent quality system regulations before market approval.
The Dermal Filler Cannula Market is a specialized segment within the broader medical aesthetics industry, dedicated to the manufacturing and distribution of specialized injection tools. These tools are crucial for the safe and effective delivery of viscoelastic substances used in cosmetic and reconstructive procedures. Cannulas, particularly the blunt-tip variants, represent a significant paradigm shift from traditional sharp hypodermic needles. This shift is rooted in the pursuit of enhanced safety, driven by clinical evidence demonstrating a substantial reduction in the incidence of complications such as hematoma formation, ecchymosis, and, most critically, inadvertent intra-arterial injection leading to potential vascular compromise or blindness. The market's robust growth trajectory is intrinsically linked to the global rise in non-surgical cosmetic interventions, reflecting a societal trend towards minimally invasive solutions that offer rapid results and short recovery times. The design innovation in cannulas allows practitioners to employ advanced techniques, such as the fanning technique, where a single entry point is used to treat a wide area, reducing patient trauma and maximizing efficiency. This efficiency is a powerful driver for high-volume aesthetic practices seeking to improve throughput and patient satisfaction scores.
Further detailed analysis of the market structure reveals that standardization across the product range, including defined gauge sizes (ranging from 18G for high-viscosity fillers down to 30G for superficial applications), standardized lengths, and hub compatibility, are critical elements affecting professional adoption. The material composition, predominantly medical-grade stainless steel, must ensure sufficient rigidity to navigate subcutaneous tissue layers while maintaining flexibility to contour effectively without kinking. Manufacturers are continually investing in research and development to create proprietary surface treatments and coatings that minimize the coefficient of friction, optimizing the gliding capability of the cannula. The competitive landscape is characterized by companies that successfully marry engineering precision with strong clinical evidence supporting improved patient outcomes. Companies focusing on comprehensive practitioner training packages, often including cadaver workshops and advanced online modules, gain a competitive edge by lowering the barrier to safe adoption among newly certified aesthetic injectors.
The segmentation by Product Type, notably the dominance of Blunt Tip/Microcannulas, highlights the market's emphasis on risk mitigation. Sharp Tip Cannulas, while still used for creating initial entry points or highly localized injections, are rapidly being superseded by their blunt counterparts for sweeping movements and area treatments. The End-User segmentation reveals that specialized Dermatology Clinics and Plastic Surgery Centers remain the largest consumers, primarily due to the complex nature of the procedures often requiring cannulas. However, the accelerating proliferation of Medical Spas, which are increasingly integrating advanced aesthetic services, signifies a dynamic expansion in the distribution network. This shift mandates that manufacturers develop robust logistical strategies capable of serving diverse clinic sizes and geographical locations, ensuring consistent supply chain integrity for sterile, single-use devices.
The economic viability of the market is also underpinned by the longevity and quality of modern dermal fillers. As fillers become more durable and capable of delivering volume augmentation lasting 12 to 24 months, the investment in high-quality delivery tools like cannulas becomes justified for practitioners seeking to protect their reputation and minimize liability associated with adverse events. Moreover, the global shift in aesthetic ideals, moving toward subtle, natural-looking enhancements rather than dramatic surgical changes, necessitates tools that allow for nuanced, layered filler placement—a technique where microcannulas excel. The interplay of technological precision, regulatory compliance, and consumer demand for safety confirms the cannula market's trajectory as a cornerstone of the non-surgical aesthetic industry. The market is highly sensitive to regulatory changes, especially regarding product safety documentation, requiring extensive post-market surveillance and rigorous manufacturing quality control, which ultimately enhances product reliability and brand trust. This rigorous approach contributes significantly to the sustained high valuation and growth forecast for the Dermal Filler Cannula Market. The high barrier to entry for new competitors ensures that market leadership remains concentrated among entities capable of sustained innovation and adherence to global medical device standards, particularly concerning sterility assurance levels and material traceability.
The segmentation based on Gauge Size is particularly important for clinical efficacy. Smaller gauges (27G, 30G) are essential for injecting low-viscosity fillers or performing delicate work in superficial layers, such as tear troughs or fine lines, where precision is paramount. Conversely, larger gauges (18G, 20G) are utilized for high-viscosity fillers or procedures requiring substantial volumetric replacement in deep tissue planes, such as mid-face lifting or body contouring applications. Manufacturers frequently offer comprehensive kits containing a variety of gauges and lengths, recognizing that practitioners require a versatile arsenal of tools to address the highly individualized needs of their patient base. This customization trend drives higher average selling prices (ASPs) for specialized, branded cannula systems compared to generic sharp needles.
Furthermore, the influence of intellectual property (IP) and patents surrounding specialized cannula tips (e.g., dome tips, specific curvature designs) plays a vital role in market differentiation. Companies possessing strong IP portfolios can maintain premium pricing and stave off immediate competition, ensuring higher profit margins. The market is not merely selling a disposable device; it is selling an enhanced safety protocol and superior technique delivery system. This inherent value proposition supports the robust market valuation and investment in continuous engineering refinement, ensuring that the next generation of cannulas will offer even lower injection forces, improved ergonomic handling, and compatibility with emerging complex biological filler materials.
The continuous professional development (CPD) element is a latent but powerful force in the market. As new cannula designs are introduced, the need for revised training material and workshops becomes mandatory. This creates a symbiotic relationship between manufacturers and aesthetic training academies, where product innovation is tied directly to the education infrastructure. For emerging markets, the accessibility of affordable, standardized training is often the bottleneck, presenting an opportunity for international manufacturers to partner with local institutions to drive adoption and ensure safe clinical practice, thereby indirectly boosting sales volumes in these high-potential regions. The environmental impact of single-use plastic and metal devices is also beginning to emerge as a consideration, prompting initial R&D into more sustainable or bio-compatible materials, although this segment is currently nascent.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
