Dextran Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 437844 | Date : Dec, 2025 | Pages : 248 | Region : Global | Publisher : MRU
Dextran Market Size
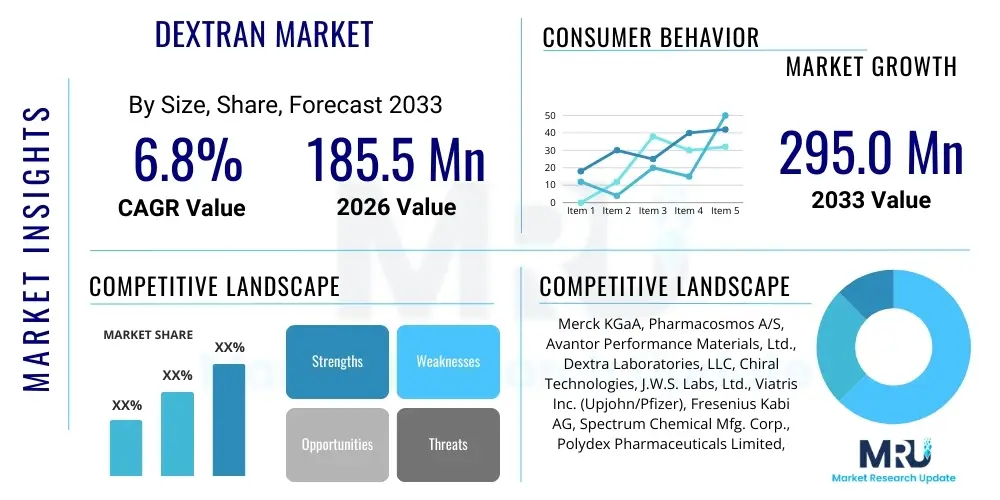
The Dextran Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2026 and 2033. The market is estimated at USD 185.5 Million in 2026 and is projected to reach USD 295.0 Million by the end of the forecast period in 2033.
Dextran Market introduction
The Dextran Market encompasses the production, distribution, and utilization of Dextran, a complex branched glucan derived from the fermentation of sucrose by certain lactic acid bacteria. Dextran is characterized by its high molecular weight and biocompatibility, making it an indispensable polymer across various high-value industries. Historically, its primary application has been as a blood volume expander (Dextran 40 and Dextran 70) in emergency and surgical settings due to its plasma-like properties and ability to reduce blood viscosity. Beyond transfusion medicine, Dextran serves critical roles as a stabilizing agent for biological products, a versatile matrix for chromatographic separation, and a key component in advanced drug delivery systems.
The product is available in various molecular weights, which dictates its functional utility. Low molecular weight dextrans (LMWDs) like Dextran 10 and 40 are preferred for microcirculation improvement and antithrombotic effects, while higher molecular weight variations are used in research and as pharmaceutical excipients. Major applications include intravenous fluids, diagnostic reagents (such as Dextran-coated iron oxide nanoparticles), and in tissue engineering scaffolds. The inherent benefits of Dextran—including low immunogenicity, high aqueous solubility, and tailored molecular architecture—drive its adoption in sensitive medical applications. Key driving factors involve the rising prevalence of chronic diseases requiring advanced drug targeting systems and the increasing global demand for safe, effective plasma volume substitutes, especially in emerging economies where healthcare infrastructure is rapidly expanding.
Dextran Market Executive Summary
The Dextran Market is positioned for robust expansion, primarily driven by accelerated utilization within the pharmaceutical and biotechnology sectors, which require high-purity, stable excipients and drug carriers. Current business trends indicate a significant shift towards customized molecular weight Dextran variants to enhance targeted drug delivery efficiency, moving beyond traditional bulk production focused solely on volume expansion. Strategic partnerships between Dextran manufacturers and specialized biotech firms are becoming common to co-develop novel diagnostic and therapeutic agents stabilized by Dextran. Furthermore, stringent regulatory scrutiny in developed markets is encouraging manufacturers to adopt advanced purification techniques, ensuring compliance with pharmacopeial standards, thus raising the overall quality threshold of available products globally.
Regional trends reveal Asia Pacific (APAC) as the fastest-growing market, propelled by massive government investment in healthcare infrastructure, particularly in countries like China and India, and the rising demand for sophisticated pharmaceutical manufacturing inputs. North America and Europe, while mature, maintain dominance in terms of value, driven by high R&D expenditure in diagnostics and personalized medicine where Dextran hydrogels and microparticles are crucial. Segment-wise, the Application segment sees the highest growth in Drug Delivery and Diagnostics, overshadowing the traditional Volume Expanders segment, reflecting a fundamental evolution of Dextran from an emergency treatment to an advanced pharmaceutical ingredient. The Dextran 40 product type remains the most critical segment by revenue due to its established use in clinical settings, though Dextran derivatives for high-tech applications are rapidly gaining market share.
AI Impact Analysis on Dextran Market
Users frequently inquire about how Artificial Intelligence (AI) can optimize the complex fermentation and purification processes used in Dextran manufacturing, asking if AI models can predict optimal molecular weight distribution or yield based on input conditions. Another key concern relates to AI’s role in accelerating Dextran derivative design for specific biomedical applications, such as improving drug targeting specificity or designing more stable hydrogel matrices. Key themes emerging from user inquiries center around operational efficiency, R&D acceleration, and quality control automation. Users expect AI to reduce batch-to-batch variability, minimize waste in high-cost purification steps like ultrafiltration, and identify novel, cost-effective raw material sources or synthesis pathways for medically complex Dextrans, ultimately ensuring a more reliable and cost-effective supply chain for pharmaceutical customers.
The deployment of AI and machine learning (ML) models is set to revolutionize Dextran synthesis by establishing predictive maintenance schedules for bioreactors and optimizing fermentation kinetics in real time. ML algorithms are being utilized to analyze high-throughput screening data, identifying the most effective Dextran modifications (e.g., sulfated or carboxymethylated Dextrans) for interaction with specific biological targets, thereby significantly shortening the development cycle for advanced drug conjugates. This integration of computational intelligence moves Dextran manufacturing from empirical experimentation toward data-driven precision, addressing major industry challenges such as purity assurance and consistency, especially for parenteral preparations.
- AI-driven optimization of microbial fermentation parameters (pH, temperature, nutrient feed rates) to maximize Dextran yield and control molecular weight distribution (MWD).
- Predictive modeling using ML to minimize batch variation and improve product consistency, crucial for pharmacopeial compliance.
- Automation of complex purification processes (chromatography, ultrafiltration) using AI for real-time quality assurance and reduced operational cost.
- AI-assisted design and simulation of Dextran-based nanocarriers and hydrogels for enhanced drug encapsulation and controlled release profiles.
- Analysis of regulatory data and market trends by AI to inform strategic investment decisions regarding high-demand Dextran derivatives.
DRO & Impact Forces Of Dextran Market
The Dextran Market is substantially influenced by the synergy between increasing healthcare expenditure globally, particularly in critical care and oncology, and the corresponding regulatory pressure to ensure product safety and efficacy. Key market Drivers include the widespread application of Dextran as an essential ingredient in intravenous solutions, the expansion of the biotechnology sector demanding high-quality excipients, and the surge in research focused on Dextran derivatives for advanced applications like gene therapy vectors and targeted imaging agents. Conversely, Restraints such as the high cost and complexity associated with achieving pharmaceutical-grade purity (especially removing endotoxins and ensuring precise MWD), along with potential supply chain disruptions affecting raw materials (sucrose), present significant challenges to market growth. These dynamics are further amplified by the inherent complexity of scaling fermentation processes while maintaining consistent product characteristics.
The primary Opportunities lie in capitalizing on the shift from synthetic polymers to natural, biocompatible alternatives in medical device coatings and tissue engineering scaffolds, where Dextran exhibits superior performance. Furthermore, the development of novel, low-cost enzymatic synthesis methods, which offer greater control over molecular structure compared to traditional microbial fermentation, represents a key avenue for market penetration and differentiation. Impact Forces include intense scrutiny from regulatory bodies like the FDA and EMA regarding the clinical safety of blood volume expanders and the necessity for rigorous clinical trials for new Dextran-based drug formulations. Technological breakthroughs in separation science (e.g., specialized membrane technology) are also impacting production efficiency, driving down manufacturing costs for high-purity grades, thereby making Dextran more competitive against synthetic alternatives in cost-sensitive markets.
Segmentation Analysis
The Dextran Market is comprehensively segmented based on its technical specifications, functional application, and final point of consumption, reflecting its diverse utility across life sciences. Product type segmentation is critical, as the molecular weight (MW) of Dextran fundamentally dictates its function, ranging from low MW Dextran 10 (used primarily in diagnostics) to medium MW Dextran 40 and 70 (essential in clinical plasma expansion). The Application segment is witnessing rapid evolution, moving focus away from mature segments like Volume Expanders towards high-growth areas such as specialized Drug Delivery systems and stability-enhancing Excipients, which command higher price points and offer greater scope for product innovation. The End-Use analysis highlights the dominance of the Pharmaceutical and Biotechnology sectors due to their need for large volumes of GMP-grade Dextran, followed by diagnostic laboratories and research institutions.
Geographic segmentation underscores the market disparity between highly regulated, research-intensive markets in North America and Europe, and rapidly expanding manufacturing and clinical markets in the Asia Pacific region. The segmentation by manufacturing process, encompassing microbial fermentation and enzymatic synthesis, illustrates the shift towards more controllable and high-yield synthesis methods, although fermentation remains the primary commercial route. Analyzing these segments provides strategic insights into which regions and applications offer the most immediate revenue potential and where technological investments are most warranted. For instance, companies focusing on advanced Drug Delivery systems are concentrating their R&D efforts on producing modified and functionalized Dextran derivatives, capitalizing on the increasing global demand for precise therapeutic targeting, particularly for cancer and chronic inflammatory diseases.
- By Product Type:
- Dextran 10 (Low Molecular Weight)
- Dextran 40 (Medium Molecular Weight)
- Dextran 70 (High Molecular Weight)
- Dextran Derivatives (e.g., Carboxymethyl Dextran, Sulfated Dextran)
- By Application:
- Blood Volume Expanders
- Drug Delivery Systems (Nanoparticles, Conjugates)
- Stabilizing Agents and Excipients
- Diagnostics and Laboratory Use (Chromatography, Cell Culture)
- Food and Cosmetics
- By End-Use:
- Pharmaceutical & Biotechnology Companies
- Hospitals and Clinics
- Academic & Research Institutes
- Diagnostic Laboratories
- By Manufacturing Process:
- Microbial Fermentation
- Enzymatic Synthesis
- By Region:
- North America (U.S., Canada)
- Europe (Germany, U.K., France, Italy, Spain)
- Asia Pacific (China, Japan, India, South Korea)
- Latin America (Brazil, Mexico)
- Middle East & Africa (MEA)
Value Chain Analysis For Dextran Market
The Dextran value chain begins with the upstream sourcing of raw materials, primarily high-purity sucrose or molasses, which are essential substrates for microbial fermentation. Efficiency at this stage is crucial as the quality of the starting material heavily influences the yield and subsequent purification complexity. The manufacturing core involves the highly specialized fermentation or enzymatic synthesis processes, followed by rigorous purification steps—including precipitation, filtration, and chromatography—necessary to achieve pharmacopeial standards (e.g., USP/EP grade Dextran). These purification steps, particularly the precise fractionation to control molecular weight distribution, represent the highest value-add stage and are a significant determinant of final product cost and market applicability.
Midstream activities involve processing and derivatization, where basic Dextran is modified (e.g., carboxymethylation, sulfation) to create specialized derivatives required for advanced drug delivery and research applications. This stage requires significant technical expertise and intellectual property. The distribution channel is bifurcated: Direct distribution is common for large pharmaceutical end-users who require bulk, customized batches and engage directly with manufacturers, ensuring strict quality control and traceability. Indirect channels, involving specialized chemical distributors or bioreagent suppliers, serve smaller research laboratories, diagnostic firms, and academic institutions, providing smaller quantities and a wider variety of standard Dextran grades. Efficiency in the logistics of handling temperature-sensitive or high-purity materials is paramount throughout the entire distribution network.
Dextran Market Potential Customers
The primary customers for Dextran are large multinational pharmaceutical and biotechnology corporations that utilize Dextran both as a primary therapeutic agent (e.g., plasma volume expanders) and as critical excipients or formulation components. These customers demand GMP-certified, high-purity Dextran derivatives for injectable drug formulations, stability enhancement of biologics (vaccines, proteins), and advanced targeted drug delivery systems, often requiring Dextran customized to specific molecular weights and functional groups. Hospitals, particularly those with critical care units and surgical departments, constitute another major end-user base, purchasing Dextran 40 and 70 for emergency fluid resuscitation and anticoagulation protocols.
A rapidly growing segment of potential buyers includes diagnostic companies and research laboratories, which require Dextran for use as a separation matrix (Sephadex), cell culture media additive, or component in diagnostic reagents and contrast agents (e.g., magnetic resonance imaging). These customers often prioritize the consistency and specific technical specifications of the Dextran, rather than sheer volume. Furthermore, specialty chemical and cosmetic manufacturers represent emerging buyers, utilizing Dextran for its thickening, moisturizing, and film-forming properties in high-end personal care products and specialized food ingredients, although this segment currently accounts for a smaller share compared to the medical and biotech sectors.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 185.5 Million |
| Market Forecast in 2033 | USD 295.0 Million |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Merck KGaA, Pharmacosmos A/S, Avantor Performance Materials, Ltd., Dextra Laboratories, LLC, Chiral Technologies, J.W.S. Labs, Ltd., Viatris Inc. (Upjohn/Pfizer), Fresenius Kabi AG, Spectrum Chemical Mfg. Corp., Polydex Pharmaceuticals Limited, Pfanstiehl, Inc., BMG Pharma S.p.A., Wuhan Fine Chemical Co., Ltd., Galenica S.A., TCI Chemicals (India) Pvt. Ltd., Tokyo Chemical Industry Co., Ltd. (TCI), Thermo Fisher Scientific Inc., C.P. PHARMACEUTICALS (INDIA) PVT. LTD., BioVision Inc., Alfa Aesar (a part of Thermo Fisher) |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Dextran Market Key Technology Landscape
The core technology underpinning the Dextran market revolves around high-yield, precision fermentation and advanced chemical modification techniques. Microbial fermentation, typically utilizing strains of Leuconostoc mesenteroides, remains the dominant commercial method, requiring sophisticated bioreactor control systems to manage culture conditions meticulously and ensure optimal synthesis of the desired crude Dextran polymer. However, the critical challenge lies in the downstream processing, where modern membrane separation technologies, such as ultrafiltration and nanofiltration, are replacing older, less efficient precipitation methods. These membrane technologies enable highly accurate fractionation of the polymer mixture, allowing manufacturers to isolate Dextran fractions with incredibly narrow and precise molecular weight distributions (MWD), which is paramount for high-grade pharmaceutical applications.
A crucial technological advancement driving market innovation is enzymatic synthesis, utilizing Dextransucrase enzymes in controlled reaction conditions. This approach offers superior control over the structural characteristics of the resulting Dextran, including the degree of branching, potentially leading to novel polymers with enhanced biological activity or stability. Furthermore, advancements in analytical instrumentation, particularly high-performance size-exclusion chromatography (HPSEC) coupled with sophisticated detectors, are vital for quality control, ensuring compliance with strict regulatory requirements concerning MWD purity and absence of contaminants. The integration of continuous manufacturing processes is also emerging as a key trend, aimed at improving scalability and reducing batch-to-batch variability compared to traditional batch fermentation.
Regional Highlights
Geographically, the Dextran market demonstrates varied dynamics driven by regional differences in healthcare spending, pharmaceutical manufacturing capacity, and regulatory environments. North America, specifically the United States, commands a significant market share by value. This dominance is attributed to high expenditure on advanced biotechnology research, the strong presence of major pharmaceutical innovators, and the intensive use of Dextran derivatives in cutting-edge diagnostics and drug delivery R&D. Stringent quality standards in this region necessitate the use of premium, highly purified Dextran grades, supporting higher average selling prices.
Europe represents a mature but stable market, characterized by established healthcare systems and robust academic research focused on biomaterials and regenerative medicine. Countries like Germany and the U.K. are leading consumers, particularly utilizing Dextran 70 as a volume expander and various derivatives in clinical trials for oncology treatments. The European market emphasizes GMP compliance and sustainability in manufacturing. Meanwhile, the Asia Pacific (APAC) region is projected to exhibit the fastest growth over the forecast period. This rapid expansion is fueled by the massive expansion of generic drug manufacturing bases in India and China, increasing local demand for plasma substitutes, and government initiatives aimed at expanding access to advanced medical treatments. APAC manufacturers are increasingly focused on improving quality to meet international standards and capture export opportunities, shifting from commodity Dextran production to specialized grades.
- North America (U.S. & Canada): Leading market in terms of R&D intensity and consumption of high-grade Dextran derivatives for oncology and biotechnology applications. Focus on precision medicine and nanomedicine utilizing functionalized Dextran.
- Europe (Germany, U.K.): Strong presence in pharmaceutical manufacturing and research. Stable demand for Dextran 70 and excipients; high regulatory focus on product traceability and quality.
- Asia Pacific (China, India): Fastest-growing region driven by expanding healthcare infrastructure, high incidence of trauma necessitating volume expanders, and rapid growth in biopharma outsourcing. Increasing investment in local high-purity production capabilities.
- Latin America (Brazil, Mexico): Moderate growth driven by improving access to critical care and increasing clinical use of blood volume expanders. Market growth dependent on stable economic conditions and local manufacturing expansion.
- Middle East & Africa (MEA): Emerging market with growth concentrated in Gulf Cooperation Council (GCC) countries due to high healthcare expenditure and modernization of medical facilities. Demand primarily for basic volume expanders and diagnostics reagents.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Dextran Market.- Pharmacosmos A/S
- Merck KGaA (MilliporeSigma)
- Avantor Performance Materials, Ltd.
- Dextra Laboratories, LLC
- Polydex Pharmaceuticals Limited
- Fresenius Kabi AG
- Viatris Inc. (Upjohn/Pfizer)
- Spectrum Chemical Mfg. Corp.
- Pfanstiehl, Inc.
- J.W.S. Labs, Ltd.
- Chiral Technologies
- BMG Pharma S.p.A.
- Wuhan Fine Chemical Co., Ltd.
- Galenica S.A.
- TCI Chemicals (India) Pvt. Ltd.
- Tokyo Chemical Industry Co., Ltd. (TCI)
- Thermo Fisher Scientific Inc.
- C.P. PHARMACEUTICALS (INDIA) PVT. LTD.
- BioVision Inc.
- Alfa Aesar (a part of Thermo Fisher)
Frequently Asked Questions
Analyze common user questions about the Dextran market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary function of Dextran in clinical settings?
The primary clinical function of Dextran, particularly Dextran 40 and 70, is acting as a plasma volume expander (artificial colloid) to restore circulatory volume following significant blood loss, trauma, or burn injuries. It helps maintain oncotic pressure and improves blood flow in the microcirculation.
How is the market segmented by molecular weight, and why is this distinction important?
The market is segmented by molecular weight (MW), commonly Dextran 10, Dextran 40, and Dextran 70. This distinction is vital because MW dictates biological activity; low MW Dextran 10 is often used in diagnostics, while Dextran 40 and 70 are critical for volume expansion and antithrombotic effects, impacting pharmacokinetics and tissue penetration.
Which region is expected to show the highest growth rate for Dextran consumption?
The Asia Pacific (APAC) region is projected to record the highest growth rate (CAGR) due to rapid expansion in pharmaceutical manufacturing, increasing access to critical care services, and substantial governmental investments in modernizing healthcare infrastructure across countries like China and India.
What are the key technical challenges in Dextran manufacturing?
Key challenges include maintaining highly precise molecular weight distribution (MWD) during purification, ensuring the complete removal of toxic endotoxins to meet pharmaceutical-grade standards, and controlling batch-to-batch consistency in microbial fermentation processes, all of which are essential for drug safety.
Beyond plasma expansion, what significant applications are driving future Dextran market growth?
Future market growth is primarily driven by advanced applications in drug delivery systems, where Dextran serves as a biodegradable carrier for targeted drug conjugation (especially chemotherapy agents), and in diagnostics, where it is used as a stabilizing agent for nano-formulations and contrast agents in medical imaging.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
- Dextran Market Size Report By Type (Dextran 20, Dextran 40, Dextran 60, Dextran 70), By Application (Solutions for Injection and Infusion, Dextran Derivative), By Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Share, Trends, Outlook and Forecast 2025-2032
- Dextran Market Statistics 2025 Analysis By Application (Solutions for Injection and Infusion, Dextran Derivative), By Type (Dextran 20, Dextran 40, Dextran 60, Dextran 70, Other), and By Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Forecast 2025 to 2032
- Dextran Market Size, Share, Trends, & Covid-19 Impact Analysis By Type (Dextran 20, Dextran 40, Dextran 60, Dextran 70, Other), By Application (Pharmaceutical, Food, Industrial), By Region - North America, Latin America, Europe, Asia Pacific, Middle East, and Africa | In-depth Analysis of all factors and Forecast 2023-2030
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
