Disposable Hemoperfusion Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 436958 | Date : Dec, 2025 | Pages : 248 | Region : Global | Publisher : MRU
Disposable Hemoperfusion Market Size
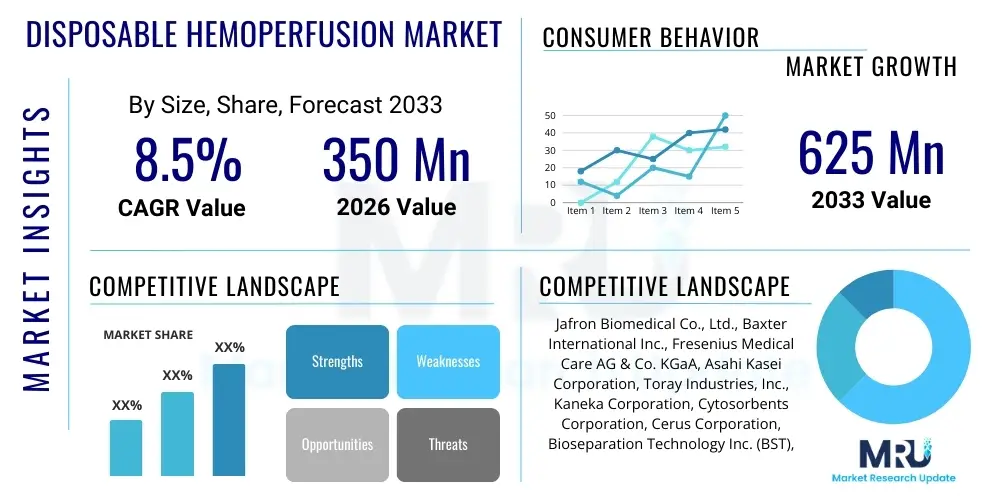
The Disposable Hemoperfusion Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.5% between 2026 and 2033. The market is estimated at USD 350 Million in 2026 and is projected to reach USD 625 Million by the end of the forecast period in 2033.
Disposable Hemoperfusion Market introduction
The Disposable Hemoperfusion Market encompasses specialized medical devices utilized for purifying blood by directly adsorbing toxic substances, metabolites, or exogenous poisons from the plasma. Hemoperfusion, a technique distinct from hemodialysis, employs cartridges containing highly biocompatible adsorbents, such as activated carbon or specific resins, to remove middle-to-large molecular weight toxins that dialysis might not efficiently clear. These single-use, disposable cartridges mitigate the risk of cross-contamination and ensure high procedural efficiency, driving their adoption in acute care settings globally. The technology is critical in treating acute poisoning, certain autoimmune disorders, and managing end-stage renal disease complications where uremic toxins accumulate.
The primary product description involves sterile, single-lumen cartridges pre-filled with adsorbent material designed for extracorporeal circulation. Major applications span intensive care units (ICUs) for drug overdose management, sepsis and septic shock treatment where inflammatory mediators need rapid removal, and hepatorenal syndrome management. The disposable nature of the product significantly streamlines hospital protocols by eliminating the need for resterilization and complex maintenance, thereby enhancing patient safety and operational workflow. Benefits include targeted removal capability, reduced treatment time compared to conventional methods for certain intoxications, and improved survival rates in cases of severe poisoning or sepsis-related organ failure.
Driving factors for market expansion include the escalating global incidence of acute drug and chemical poisoning, increasing awareness regarding the efficacy of hemoperfusion in critical care, and substantial advancements in adsorbent material science, leading to higher selectivity and compatibility. Furthermore, regulatory support for innovative blood purification therapies, particularly in treating inflammatory conditions like cytokine storm associated with severe infections, propels market demand. Investment in R&D focusing on next-generation adsorbents that target specific biomarkers, coupled with expanding reimbursement policies across developed economies, solidify the market's positive trajectory.
Disposable Hemoperfusion Market Executive Summary
The Disposable Hemoperfusion Market exhibits robust growth, primarily fueled by shifting global healthcare priorities toward effective critical care interventions and the rising prevalence of chronic kidney disease and intoxication cases. Business trends indicate a strong focus on strategic mergers and acquisitions among key players to consolidate technological expertise and expand geographic footprint, particularly targeting high-growth economies in Asia Pacific. Manufacturers are increasingly investing in clinical trials demonstrating the utility of hemoperfusion in novel applications, such as cardiovascular disease management and neurodegenerative conditions, moving beyond traditional applications like renal failure and poisoning. The competitive landscape is intensifying, necessitating continuous innovation in cartridge design and adsorbent chemistry to secure market share and differentiation.
Regionally, North America and Europe maintain dominance, characterized by high healthcare expenditure, established critical care infrastructure, and favorable reimbursement scenarios for blood purification therapies. However, the Asia Pacific region is poised to register the highest CAGR, driven by the massive patient pool, improving accessibility to advanced medical technologies, and rapid development of modern healthcare facilities in countries like China, India, and South Korea. These regions present substantial opportunities for market penetration through localized manufacturing and distribution partnerships, addressing the growing demand for effective detoxification solutions. Regulatory harmonization across various jurisdictions remains a critical factor influencing the speed of product introduction and commercialization in emerging markets.
Segment trends highlight the substantial market share held by activated carbon-based cartridges due to their broad-spectrum detoxification capabilities and cost-effectiveness. However, the specialized resin adsorbent segment is projected to experience accelerated growth, driven by demand for highly selective removal of specific molecules, such as inflammatory mediators (cytokines) in sepsis treatment or bilirubin in hepatic failure. Furthermore, the application segment focused on critical care units, particularly acute poisoning, remains the largest, but the sepsis management application is rapidly expanding due to global emphasis on reducing sepsis mortality. Customization and integration of these disposable systems with existing dialysis or filtration platforms are key technological trends influencing segment performance and adoption rates.
AI Impact Analysis on Disposable Hemoperfusion Market
User queries regarding the impact of Artificial Intelligence (AI) on the Disposable Hemoperfusion Market primarily center on optimizing treatment protocols, predicting patient response, and refining adsorbent material development. Key themes include how AI algorithms can personalize hemoperfusion dosage and duration based on real-time toxin levels, and the potential for machine learning to accelerate the discovery of novel, highly selective adsorbent polymers. Users are concerned with the integration challenge between AI-powered predictive diagnostics and existing extracorporeal systems. Expectations are high regarding AI’s ability to move hemoperfusion from a generalized detoxification method to a precision therapeutic tool, potentially reducing treatment variability and improving clinical outcomes by identifying the optimal timing for intervention in complex syndromes like septic shock or cytokine release syndrome (CRS).
- AI-driven optimization of hemoperfusion timing and duration based on real-time patient physiological data and circulating toxin levels.
- Machine Learning (ML) acceleration in discovering and synthesizing novel, highly selective adsorbent materials for targeted toxin removal.
- Integration of AI-powered predictive analytics to identify patients at high risk of sepsis progression, enabling proactive initiation of hemoperfusion therapy.
- Enhancement of manufacturing quality control and efficiency through AI-based visual inspection and anomaly detection in disposable cartridge production.
- Development of smart hemoperfusion devices incorporating AI for automated pressure monitoring and early detection of circuit complications, improving patient safety.
DRO & Impact Forces Of Disposable Hemoperfusion Market
The Disposable Hemoperfusion Market is characterized by strong fundamental drivers rooted in the rising global burden of critical illnesses, offset by significant regulatory hurdles and cost constraints in developing economies. The primary driving force is the proven clinical efficacy of hemoperfusion in acute settings where rapid detoxification is mandatory, such as acute drug poisoning and the increasingly recognized role in managing complex inflammatory responses like sepsis-induced cytokine storm. Restraints include the high capital investment required for establishing widespread hemoperfusion centers, coupled with fragmented or inadequate reimbursement policies in certain developing healthcare systems. Opportunities are centered on leveraging advancements in nanotechnology and material science to create bio-selective adsorbents, opening avenues for application in chronic diseases and autoimmune conditions, which represents a vast untapped market potential.
Drivers: The increasing incidence of acute renal failure (ARF) and severe intoxication worldwide significantly drives demand for efficient extracorporeal treatments. Furthermore, the growing adoption of hemoperfusion as an adjunctive therapy in sepsis management, following updated clinical guidelines supporting early aggressive intervention, provides substantial market momentum. Technological innovation focused on improving the biocompatibility of adsorbents and reducing treatment-related complications, such as platelet consumption, also fuels adoption. The shift towards disposable systems, ensuring sterility and ease of use, aligns perfectly with modern hospital operational demands, minimizing infection risks associated with reuse.
Restraints: Key restraints include the overall high procedural costs associated with single-use disposable cartridges and the specialized training required for clinical staff to operate hemoperfusion machines effectively. Moreover, limited robust, large-scale clinical evidence supporting the efficacy of hemoperfusion across all potential applications (especially chronic conditions) can sometimes hinder broader clinical acceptance and complicate reimbursement negotiation processes. Regulatory scrutiny regarding the safety and leachables profile of adsorbent materials adds complexity to the product approval lifecycle, potentially slowing down the commercialization of new technologies.
Opportunities: Significant opportunities arise from the ongoing research into applying hemoperfusion for chronic conditions, including the removal of beta-2 microglobulin in long-term dialysis patients and modulation of immune responses in autoimmune disorders. Emerging markets, particularly in APAC and Latin America, offer immense growth potential due to expanding healthcare infrastructure and rising disposable incomes allowing for access to advanced therapies. Developing point-of-care, miniaturized hemoperfusion systems that are easier to deploy in non-hospital settings or smaller clinics represents a critical long-term opportunity for market expansion and accessibility. Strategic partnerships between device manufacturers and pharmaceutical companies exploring combination therapies also hold promise.
Segmentation Analysis
The Disposable Hemoperfusion Market is meticulously segmented based on adsorbent type, application, and end-user, providing a granular view of market dynamics and adoption patterns. The segmentation by adsorbent material is critical as it dictates the detoxification capability and selectivity of the device, influencing clinical use. Activated carbon remains dominant due to its general adsorption properties, but polymer and resin-based adsorbents are gaining traction for targeted molecular removal. Application segmentation highlights the primary clinical domains utilizing this technology, with acute poisoning and critical care management commanding the largest shares, reflecting the immediate necessity for rapid blood purification in these settings. End-user analysis focuses predominantly on hospital settings, particularly ICUs and nephrology departments, which are the primary centers for administering extracorporeal blood purification treatments.
- By Adsorbent Type:
- Activated Carbon
- Polymer Adsorbents (e.g., Synthetic Resins)
- Neutral Macroporous Resins
- Specific Immunoadsorbents
- By Application:
- Acute Poisoning and Overdose
- Sepsis and Septic Shock Management (Cytokine Adsorption)
- Acute-on-Chronic Liver Failure (AOCLF)
- Drug Removal (Chemotherapy Agents, others)
- Kidney Failure and Uremic Toxin Removal
- By End User:
- Hospitals (ICUs, Nephrology Departments)
- Dialysis Centers and Clinics
- Ambulatory Surgical Centers
- By Region:
- North America
- Europe
- Asia Pacific (APAC)
- Latin America (LATAM)
- Middle East & Africa (MEA)
Value Chain Analysis For Disposable Hemoperfusion Market
The value chain for the Disposable Hemoperfusion Market starts with the sourcing of highly specialized raw materials, primarily medical-grade plastics for cartridge casing and biocompatible adsorbent materials (activated carbon, proprietary resins). Upstream activities involve intensive R&D focused on synthesizing and functionalizing these adsorbents to achieve optimal surface area, porosity, and molecular selectivity while ensuring zero leachables. This phase requires strong collaboration with specialized chemical and material science suppliers. The manufacturing stage, which includes highly precise assembly and sterilization of the disposable cartridges under stringent regulatory oversight (ISO, FDA, CE), represents the highest value addition due to the complexity of quality control and cleanroom requirements. Effective management of intellectual property related to adsorbent chemistry is crucial at this stage.
Downstream analysis focuses on distribution and logistics, which must handle sterile, temperature-sensitive medical devices efficiently. Distribution channels are predominantly indirect, relying heavily on specialized medical device distributors and wholesalers who maintain deep relationships with critical care units and hospital procurement teams. Direct sales are typically reserved for large group purchasing organizations (GPOs) or major hospital networks where high-volume contracts are negotiated. The final stage involves the clinical usage by nephrologists, intensivists, and critical care nurses, followed by waste disposal of the single-use cartridge, adhering to strict biohazard waste regulations. Marketing efforts are heavily focused on clinical education and demonstrating favorable pharmacoeconomic data to gain acceptance among healthcare providers and secure favorable reimbursement codes.
The shift towards indirect distribution is prominent, particularly in global markets, allowing manufacturers to leverage local expertise regarding import regulations and regional sales tactics. However, maintaining close communication with end-users through direct channels remains vital for gathering feedback essential for product iteration and technological upgrades. Key challenges in the value chain include managing supply chain resilience for highly specialized raw materials and navigating complex global regulatory approval processes. Streamlining the logistics for rapid delivery to acute care settings, where timely access to hemoperfusion devices can be life-saving, is a critical operational component affecting the overall efficiency of the market.
Disposable Hemoperfusion Market Potential Customers
The primary customers and end-users of disposable hemoperfusion cartridges are institutional healthcare providers requiring specialized detoxification and blood purification capabilities. Hospitals, particularly those with large Intensive Care Units (ICUs) and dedicated Nephrology Departments, represent the largest customer segment due to the necessity of treating acute conditions such as drug overdoses, severe intoxications, and complications arising from kidney failure or liver dysfunction. The high volume of critical care admissions, where immediate intervention is crucial, dictates a continuous demand for these disposable systems. Academic medical centers and large tertiary care hospitals, often involved in clinical trials and early adoption of advanced therapies, are key opinion leaders and high-volume purchasers.
Secondary but rapidly growing potential customers include specialized Dialysis Centers and independent Nephrology Clinics that are expanding their service offerings beyond conventional hemodialysis to include adjunct purification therapies for managing chronic disease complications or specific uremic toxins. The integration of hemoperfusion into routine care pathways for maintenance dialysis patients who exhibit high inflammatory markers or specific cardiovascular risk factors is becoming more prevalent, widening the customer base beyond strictly acute care. Furthermore, governmental healthcare providers and large military medical facilities, often needing robust solutions for mass casualty events or exposure to chemical agents, also constitute a significant purchasing segment.
Ultimately, the procurement decisions are influenced by clinical outcomes data, the ease of integration with existing extracorporeal circulation platforms, and the total cost of ownership, including the cost of the disposable cartridge and associated operational expenses. Biomedical engineers and procurement managers within hospital groups often prioritize suppliers offering robust educational support and systems that minimize complexity for nursing staff. Therefore, successful market penetration relies heavily on generating strong clinical evidence and providing reliable, user-friendly disposable systems that demonstrate clear patient benefit and cost-effectiveness in managing high-acuity conditions.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 350 Million |
| Market Forecast in 2033 | USD 625 Million |
| Growth Rate | 8.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Jafron Biomedical Co., Ltd., Baxter International Inc., Fresenius Medical Care AG & Co. KGaA, Asahi Kasei Corporation, Toray Industries, Inc., Kaneka Corporation, Cytosorbents Corporation, Cerus Corporation, Bioseparation Technology Inc. (BST), Haidilao Medical, Aferetica S.p.A., HemoCleanse Technologies, Inc., Medica S.p.A., PuriBlood Medical Co., Ltd., HealthLife, Inc., AWAK Technologies, Inc., Infomed S.R.L. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Disposable Hemoperfusion Market Key Technology Landscape
The Disposable Hemoperfusion Market is heavily influenced by advancements in adsorbent chemistry, primarily focusing on improving biocompatibility and enhancing the specificity of toxin removal. The current landscape is dominated by sophisticated adsorption columns utilizing high-surface-area materials, including modified activated carbons and synthetic polymer resins. Activated carbon technologies have evolved to incorporate protective biocompatible coatings, such as cellulose or hydrogel membranes, which minimize contact activation of blood components (like platelets and coagulation cascade factors) while maintaining high adsorption capacity for medium to large molecular weight toxins and drug compounds. This surface modification is critical for reducing adverse events and extending treatment duration safely.
A significant technological driver is the development of selective sorbents, often proprietary polymeric or immunoadsorbents, designed to target specific pathological mediators. Examples include macroporous resin cartridges engineered specifically for removing bilirubin in liver failure or highly specialized devices for cytokine adsorption used in managing severe inflammatory states like sepsis or post-cardiac surgery syndrome. These technologies represent a shift from non-specific detoxification towards targeted therapeutic intervention, requiring complex material science engineering to ensure high binding affinity for the target molecule without significant non-specific protein removal or nutrient loss. The disposable nature of these devices necessitates cost-effective yet high-precision manufacturing techniques, often involving automated filling and sealing under aseptic conditions.
Furthermore, integration technologies that allow disposable hemoperfusion cartridges to interface seamlessly with existing continuous renal replacement therapy (CRRT) machines or standard dialysis equipment are pivotal for broad market acceptance. Innovations are also focused on miniaturization and creating cartridge designs that maximize blood flow dynamics and contact time, thereby optimizing detoxification efficiency while reducing extracorporeal blood volume requirements. The development of smart cartridges equipped with sensors for monitoring adsorption saturation levels or detecting leaks is a nascent trend that, coupled with AI integration, aims to further enhance the safety and personalized efficacy of disposable hemoperfusion procedures in critical care environments.
Regional Highlights
- North America: North America holds a leading position in the Disposable Hemoperfusion Market, driven by high prevalence of acute kidney injury, advanced critical care infrastructure, and widespread adoption of specialized blood purification techniques, especially in treating drug intoxication and septic shock. Favorable reimbursement policies, extensive clinical research activity, and the strong presence of major market players (such as Baxter and Cytosorbents) propel market growth. The U.S. remains the largest contributor, with continuous investment in technologies targeting complex critical illnesses like multi-organ failure.
- Europe: Europe represents a mature market, strongly supported by established healthcare systems, rigorous quality standards for medical devices (CE marking), and increasing clinical acceptance of hemoperfusion as a standard therapy for acute poisoning and cytokine removal. Countries such as Germany, France, and the UK are key markets, benefiting from proactive regulatory bodies and collaborative clinical networks. The focus here is increasingly on utilizing targeted immunoadsorption and resin-based systems for autoimmune diseases and complex inflammatory syndromes, broadening the application base beyond traditional detoxification.
- Asia Pacific (APAC): APAC is anticipated to exhibit the fastest growth rate globally due to rapidly developing healthcare infrastructure, increasing penetration of insurance coverage, and a large patient population suffering from chronic diseases and accidental intoxication. Government initiatives in countries like China and India to modernize critical care units and increase access to advanced treatments are major catalysts. Local manufacturing capabilities are also expanding rapidly, often offering competitive pricing, making advanced disposable hemoperfusion devices more accessible throughout the region.
- Latin America (LATAM): The LATAM region presents significant growth potential, albeit from a lower base, with increasing public and private healthcare investment aiming to improve critical care standards. Market expansion is currently constrained by budget limitations and fragmented reimbursement, but rising awareness of sepsis management protocols is driving demand for effective blood purification solutions in major economies like Brazil and Mexico. Strategic distribution partnerships are essential for overcoming logistical and financial hurdles.
- Middle East & Africa (MEA): The MEA region shows steady growth, concentrated in high-income Gulf Cooperation Council (GCC) countries (UAE, Saudi Arabia) which possess world-class healthcare facilities. Demand is driven by medical tourism and high prevalence of lifestyle-related diseases leading to acute complications. However, market adoption remains highly varied, with sub-Saharan Africa facing significant challenges related to infrastructural limitations and procurement capacity.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Disposable Hemoperfusion Market.- Jafron Biomedical Co., Ltd.
- Baxter International Inc.
- Fresenius Medical Care AG & Co. KGaA
- Asahi Kasei Corporation
- Toray Industries, Inc.
- Kaneka Corporation
- Cytosorbents Corporation
- Cerus Corporation
- Bioseparation Technology Inc. (BST)
- Haidilao Medical
- Aferetica S.p.A.
- HemoCleanse Technologies, Inc.
- Medica S.p.A.
- PuriBlood Medical Co., Ltd.
- HealthLife, Inc.
- AWAK Technologies, Inc.
- Infomed S.R.L.
- Gambro (a subsidiary of Baxter)
- Essen Technology Co., Ltd.
- Nikkiso Co., Ltd.
Frequently Asked Questions
Analyze common user questions about the Disposable Hemoperfusion market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary difference between hemoperfusion and hemodialysis?
Hemoperfusion utilizes adsorption, where blood flows over a sorbent material (like activated carbon or resin) to directly remove toxins and large molecules based on surface attraction. Hemodialysis, conversely, primarily uses diffusion and ultrafiltration across a semipermeable membrane to remove water-soluble low molecular weight substances and excess fluid.
Which clinical application drives the highest demand for disposable hemoperfusion devices?
The highest demand is driven by acute poisoning and critical care management, specifically for rapid removal of toxins, drugs, and endogenous poisons that cause life-threatening intoxication. Increasingly, sepsis and septic shock management, focusing on cytokine adsorption, represents the fastest-growing application segment.
How does the disposable nature of these cartridges influence market growth?
The disposable design significantly boosts market growth by eliminating cross-contamination risks, ensuring sterility, reducing the need for complex machine reprocessing, and streamlining critical care workflows, thereby aligning with stringent patient safety protocols and hospital operational efficiencies.
What are the key technological advancements expected in adsorbent materials?
Future advancements are focused on developing highly selective adsorbents, often proprietary synthetic resins or immunoadsorbents, capable of targeting specific pathological molecules such as inflammatory cytokines, specific drug metabolites, or autoantibodies, moving the therapy toward personalized medicine.
What are the main regional growth opportunities for the disposable hemoperfusion market?
Asia Pacific (APAC) presents the most significant growth opportunity, fueled by expanding critical care infrastructure, increasing incidence of chronic diseases requiring advanced detoxification, and improving market access and reimbursement in major economies like China and India.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
