Dissolvable Microneedle Transdermal Drug Delivery Technology Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 435338 | Date : Dec, 2025 | Pages : 242 | Region : Global | Publisher : MRU
Dissolvable Microneedle Transdermal Drug Delivery Technology Market Size
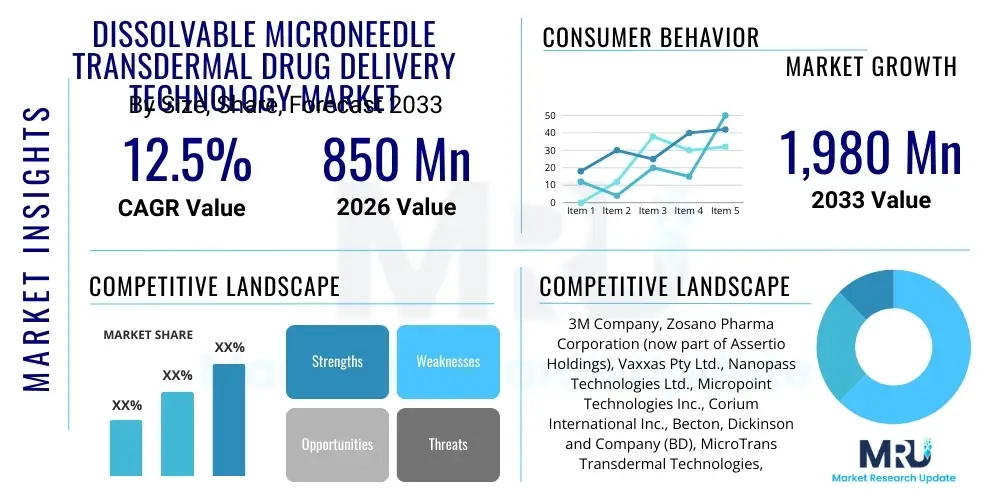
The Dissolvable Microneedle Transdermal Drug Delivery Technology Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 12.5% between 2026 and 2033. The market is estimated at USD 850 Million in 2026 and is projected to reach USD 1,980 Million by the end of the forecast period in 2033.
Dissolvable Microneedle Transdermal Drug Delivery Technology Market introduction
The Dissolvable Microneedle Transdermal Drug Delivery Technology Market encompasses innovative systems utilizing arrays of micron-sized needles made of biodegradable polymers or drug-polymer composites. These needles pierce the outermost layer of the skin (stratum corneum) painlessly to facilitate the controlled delivery of therapeutic agents directly into the epidermis or dermis, subsequently dissolving upon contact with interstitial fluid. This technology represents a paradigm shift from traditional injection methods and passive transdermal patches, offering enhanced patient compliance due to its minimally invasive nature and eliminating the need for cold chain storage for certain biologics, thereby expanding accessibility.
The primary applications of dissolvable microneedle technology span across vaccination (especially for seasonal influenza and infectious diseases), dermatology, cosmetics, and the systemic delivery of peptides, proteins, and small molecule drugs that typically face poor bioavailability through oral routes. Key benefits driving adoption include improved drug stability, precise dosing control, and bypass of first-pass metabolism. Furthermore, the technology significantly reduces the risk of accidental needle stick injuries, making it ideal for self-administration settings. This innovation addresses crucial unmet needs in drug delivery, particularly for high molecular weight compounds previously restricted to intravenous or subcutaneous injections.
Driving factors underpinning the robust market expansion include the surging global demand for non-invasive drug delivery systems, increasing prevalence of chronic diseases requiring long-term medication adherence, and substantial research and development investment by pharmaceutical companies focused on life cycle management and novel drug formulations. Regulatory bodies are increasingly supportive of these innovative platforms, further accelerating clinical trials and subsequent market approvals. The continuous advancements in materials science, particularly in biocompatible polymers and sophisticated microneedle fabrication techniques (such as molding and lithography), are instrumental in realizing the commercial potential of this technology across diverse therapeutic areas.
Dissolvable Microneedle Transdermal Drug Delivery Technology Market Executive Summary
The Dissolvable Microneedle Transdermal Drug Delivery Technology Market is characterized by vigorous innovation and strategic collaborations between specialized technology developers and large pharmaceutical enterprises seeking to optimize drug administration routes. Current business trends heavily favor platform licensing and co-development agreements, particularly focusing on vaccine delivery to improve global immunization rates, driven by the success demonstrated during pandemic responses. Segment trends indicate that the application of therapeutics, including monoclonal antibodies and chronic disease management drugs, is rapidly catching up with the established dermatology and aesthetics segments. Furthermore, the development of integrated diagnostic functionalities within microneedle patches represents a crucial technological shift, positioning these devices as not just delivery systems but comprehensive point-of-care tools.
Regional trends reveal that North America continues to dominate the market, primarily due to well-established research infrastructure, high healthcare expenditure, and the presence of numerous key market players actively engaged in clinical trials for novel microneedle products. However, the Asia Pacific region (APAC) is projected to exhibit the highest growth rate (CAGR), fueled by expanding healthcare access, governmental support for domestic biotechnology innovation in countries like China and South Korea, and a large patient pool seeking convenient drug administration methods. Regulatory harmonization efforts are beginning to facilitate faster market entry across key geographies, enhancing the global commercial landscape for dissolvable microneedle technology.
In terms of segment performance, the product type segment is witnessing a shift towards customized, dose-specific arrays, moving beyond standardized patches. The therapeutic applications segment is dominated by endocrine disorders (insulin delivery) and pain management, although oncology and immunotherapeutics are emerging as high-potential growth areas. The competitive environment remains highly dynamic, characterized by intense focus on optimizing needle geometry for deeper penetration and faster dissolution kinetics, ensuring maximum drug efficacy while maintaining the core benefit of patient comfort. Successful market penetration hinges on demonstrating cost-effectiveness and scalability in manufacturing processes, transitioning from pilot-scale production to mass commercial output necessary for widespread pharmaceutical adoption.
AI Impact Analysis on Dissolvable Microneedle Transdermal Drug Delivery Technology Market
Users frequently inquire about how Artificial Intelligence (AI) can revolutionize the design, manufacturing, and personalized application of dissolvable microneedle patches. Key themes revolve around AI's ability to optimize drug loading and release kinetics by predicting polymer-drug interactions, a significant technical hurdle in formulation development. Users are also highly interested in AI-driven predictive modeling for clinical trial outcomes, assessing patient variability in skin permeability, and tailoring microneedle characteristics (length, density, shape) to individual physiological parameters for maximized efficacy and minimized adverse effects. Furthermore, concerns often address the integration of AI-powered quality control systems in high-throughput manufacturing to ensure uniformity and safety across millions of microneedle units, a vital requirement for widespread pharmaceutical approval and adoption.
The integration of AI is expected to significantly shorten the development lifecycle of novel microneedle formulations. Machine learning algorithms can analyze vast datasets concerning polymer degradation rates, drug solubility profiles, and skin barrier properties to swiftly identify optimal material combinations and patch architectures. This predictive capability reduces the need for extensive, iterative physical testing, resulting in substantial cost and time savings. For instance, AI can model the impact of environmental factors (humidity, temperature) on patch stability, ensuring that the dissolvable matrix maintains structural integrity until application while dissolving rapidly once in contact with skin interstitial fluid, guaranteeing predictable drug delivery.
In clinical and commercial application, AI promises advanced personalization capabilities. By analyzing patient data, including skin thickness, age, and target disease state, AI can recommend or even instruct an automated manufacturing system to produce a microneedle array specifically calibrated for that individual’s needs. This level of precision, termed 'pharmacomics delivery,' enhances therapeutic outcomes, especially for highly potent or narrow therapeutic window drugs. Moreover, AI aids in post-market surveillance by analyzing user feedback and real-world efficacy data from smart patches (integrated with micro-sensors), providing continuous optimization loops for product improvement and proactive identification of potential manufacturing drifts or efficacy issues.
- AI-driven optimization of polymer selection and drug encapsulation efficiency.
- Machine learning models predicting optimal microneedle geometry for specific patient demographics and target tissues.
- Enhanced quality control systems utilizing computer vision for high-throughput defect detection in manufacturing.
- Predictive analytics to accelerate preclinical testing and forecast clinical trial success rates based on formulation variables.
- Development of smart, connected microneedle patches utilizing AI for monitoring adherence and real-time drug release confirmation.
DRO & Impact Forces Of Dissolvable Microneedle Transdermal Drug Delivery Technology Market
The Dissolvable Microneedle Transdermal Drug Delivery Market is propelled by compelling drivers such as the escalating demand for self-administered, pain-free drug delivery options and the intrinsic challenges associated with delivering large biomolecules (like vaccines and antibodies) via conventional methods. However, the market faces significant restraints, primarily centered around complex manufacturing scalability—moving from laboratory-scale casting or molding techniques to pharmaceutical-grade mass production requires substantial capital investment and stringent process validation. Opportunities abound in expanding applications beyond dermatology into systemic chronic disease management, and in exploiting patent expiries of major injectable biologics by offering superior transdermal alternatives. These forces collectively shape the market's trajectory, leading to high investment in automation and regulatory affairs compliance.
Drivers: A primary driver is the demonstrable improvement in patient adherence, especially among pediatric and geriatric populations who often exhibit needle phobia or difficulty with complex oral regimens. The enhanced thermostability offered by encapsulating drugs within a solid microneedle matrix—often eliminating the need for cold chain logistics—is a massive catalyst for global health initiatives, particularly vaccination programs in developing regions. Furthermore, the ability of these systems to overcome the limitations of the stratum corneum barrier, thereby enabling the transdermal delivery of hydrophilic and large macromolecular drugs, opens up significant market potential previously inaccessible to traditional patches.
Restraints: Despite the benefits, significant technical and economic restraints impede rapid adoption. Regulatory pathways for combination products (drug and device) are often convoluted and vary significantly across jurisdictions, slowing down commercialization. The high initial capital expenditure required for specialized cleanroom manufacturing facilities and quality control systems poses a barrier to entry for smaller biotech firms. Moreover, ensuring consistent drug loading homogeneity and confirming that the needle arrays penetrate the skin uniformly across different users remains a persistent technical challenge that must be addressed before global pharmaceutical giants fully commit to broad portfolio integration.
Opportunities & Impact Forces: The greatest opportunities lie in creating next-generation vaccine delivery platforms that offer superior immunogenicity compared to intramuscular injections, allowing for dose sparing. The development of microneedles tailored for personalized medicine, where specific drug combinations or doses are required, also presents a lucrative niche. Impact forces such as technological advancement in materials science (e.g., stimuli-responsive polymers) are high, constantly improving the speed and completeness of needle dissolution. Conversely, the high bargaining power of pharmaceutical buyers, who demand robust clinical efficacy and guaranteed scalability, acts as a constraining force on technology developers, necessitating stringent validation and quality assurance protocols to secure major licensing deals.
Segmentation Analysis
The Dissolvable Microneedle Transdermal Drug Delivery Technology Market is strategically segmented based on factors including product type, material, application, and end-user, allowing for a targeted assessment of growth pockets and competitive positioning. The segmentation highlights the market’s reliance on advanced materials such as hyaluronic acid and specialized polymers which are central to the 'dissolving' aspect of the technology, differentiating it from solid or coating-based microneedle approaches. Analysis across these segments reveals a trend towards therapeutics and vaccine delivery dominating revenue contribution, driven by high-value treatments and global immunization efforts, while the consumer health segment (dermatology and cosmetics) provides volume growth and rapid product cycles.
In terms of application, the focus has shifted from local skin treatments to systemic delivery of complex biologics. This pivotal change is supported by increasing clinical evidence demonstrating effective drug transport across the skin barrier for molecules previously considered unsuitable for transdermal administration. Furthermore, the end-user segmentation clearly indicates that pharmaceutical and biotechnology companies are the primary drivers of investment and consumption, utilizing the technology to enhance their pipelines, improve drug stability, and secure intellectual property advantages through novel delivery systems, thereby securing a strong market position for patented drugs facing generic competition.
The inherent versatility of dissolvable microneedle platforms allows for rapid pivot based on therapeutic needs. For instance, while high molecular weight drugs require deeper needle penetration and slower dissolution, vaccine delivery often utilizes arrays designed for rapid release into the dendritic cell-rich upper dermis. This adaptability means manufacturers must invest heavily in flexible, multi-purpose fabrication infrastructure. The evolving regulatory landscape, which is gradually providing clearer guidance for combination products, is expected to standardize these segments, leading to clearer product differentiation based on dissolution time and targeted anatomical site.
- By Product Type:
- Dissolvable Microneedle Patches
- Dissolvable Microneedle Arrays
- Integrated Dissolvable Microneedle Systems (with applicators)
- By Material:
- Hyaluronic Acid
- Poly Lactic-co-Glycolic Acid (PLGA)
- Carboxymethylcellulose (CMC)
- Other Biodegradable Polymers
- By Application:
- Vaccine Delivery (Influenza, COVID-19, others)
- Therapeutic Drug Delivery
- Pain Management
- Endocrine Disorders (Diabetes, Growth Hormone)
- Oncology
- Immunology
- Others
- Dermatology and Cosmetics
- By End-User:
- Pharmaceutical and Biotechnology Companies
- Hospitals and Clinics
- Homecare Settings
- By Region:
- North America
- Europe
- Asia Pacific (APAC)
- Latin America (LATAM)
- Middle East & Africa (MEA)
Value Chain Analysis For Dissolvable Microneedle Transdermal Drug Delivery Technology Market
The Value Chain for the Dissolvable Microneedle Market is complex, beginning with highly specialized upstream activities involving polymer and excipient suppliers. These suppliers must provide ultra-high purity, biocompatible, and pharmacologically inert materials suitable for microneedle fabrication techniques such as micro-molding or drawing lithography. Research and Development (R&D) is the most critical value-adding step upstream, focusing on optimizing drug formulation stability within the solid matrix and ensuring controlled, predictable release kinetics upon dissolution. Specialized contract development and manufacturing organizations (CDMOs) often play a crucial intermediary role, translating R&D prototypes into scalable manufacturing processes compliant with Good Manufacturing Practices (GMP).
Midstream activities involve the actual manufacturing, quality assurance, packaging, and sterilization processes, which require proprietary equipment and highly controlled environments. Ensuring uniformity across millions of microscopic needles, maintaining sterility without compromising drug potency, and attaching the array to an adhesive patch backing are significant operational hurdles. Downstream, the value chain shifts toward distribution and market penetration. Distribution channels are typically bifurcated: direct channels involve large pharmaceutical companies distributing approved therapeutic patches directly to hospitals, clinics, and pharmacies, often supported by integrated applicators or auto-injectors specifically designed for the patch.
Indirect distribution often characterizes the consumer health and cosmetic segments, where products move through distributors, specialized retailers, and increasingly, direct-to-consumer e-commerce platforms. The success of downstream activities relies heavily on market education and demonstrating user-friendliness, particularly for products intended for self-administration in home care settings. Key profitability resides in the R&D and intellectual property surrounding the formulation and manufacturing process, as the material cost itself, while significant for advanced polymers, is often outweighed by the value added through enhanced drug efficacy and patent protection afforded by the novel delivery route.
Dissolvable Microneedle Transdermal Drug Delivery Technology Market Potential Customers
The primary cohort of potential customers for dissolvable microneedle technology includes large multinational Pharmaceutical and Biotechnology Corporations. These entities are consistently seeking innovative delivery methods to extend the patent life of blockbuster drugs, differentiate biosimilars, or deliver challenging biological drugs that require parenteral administration. They represent the largest buyers due to their need for platform licensing, large-scale supply contracts, and integration of the technology into their existing drug pipelines for chronic conditions like diabetes, multiple sclerosis, and rheumatoid arthritis, where patient self-administration is highly desirable. Their purchasing decisions are driven by clinical trial success, scalability guarantees, and intellectual property strength.
A secondary, rapidly expanding customer base includes specialized Vaccine Manufacturers and Public Health Organizations (such as WHO or governmental agencies). These customers prioritize ease of use, stability (eliminating cold chain dependency), and high-volume manufacturing capability. Dissolvable microneedle patches offer significant logistical advantages for mass vaccination campaigns in low and middle-income countries, simplifying administration and reducing reliance on trained healthcare personnel. This segment focuses on specific applications like seasonal flu, measles, and emerging infectious disease vaccines, prioritizing global accessibility and logistical efficiency over premium pricing.
The tertiary, but highly visible, customer segment comprises Dermatology Clinics, Medical Spas, and Cosmeceutical Companies. These customers utilize the technology for local drug delivery in aesthetic treatments (e.g., anti-aging peptides, hyaluronic acid fillers) and localized dermatological therapies. Their purchasing patterns are highly influenced by consumer trends, rapid product innovation cycles, and branding, often prioritizing immediate visible results and non-medical certification over extensive regulatory approval, compared to systemic drug delivery customers. Homecare settings, served through retail pharmacies and e-commerce, also form a critical end-user group for self-administered chronic care and consumer applications.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 850 Million |
| Market Forecast in 2033 | USD 1,980 Million |
| Growth Rate | 12.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | 3M Company, Zosano Pharma Corporation (now part of Assertio Holdings), Vaxxas Pty Ltd., Nanopass Technologies Ltd., Micropoint Technologies Inc., Corium International Inc., Becton, Dickinson and Company (BD), MicroTrans Transdermal Technologies, Cosmed Pharmaceutical Co., Ltd., LTS Lohmann Therapie-Systeme AG, Nitto Denko Corporation, Kindeva Drug Delivery, Inc., PharmaTher Inc., MyLife Technologies B.V., Debiotech SA, Small Pharma Inc., Merck KGaA, Cipla Inc., Verndari Inc., Takeda Pharmaceutical Company Limited. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Dissolvable Microneedle Transdermal Drug Delivery Technology Market Key Technology Landscape
The technological landscape of the dissolvable microneedle market is defined by several sophisticated fabrication methods essential for achieving the required micron-scale precision and material consistency. The dominant technologies include replica molding, droplet-based micro-dispensing, and photolithography. Replica molding, often using polydimethylsiloxane (PDMS) molds, is widely favored for its scalability and ability to create high-density arrays with specific tip geometries. This technique allows for precise control over needle height and shape, crucial factors determining the successful penetration depth into the skin and subsequent dissolution rate. Advances in automated molding systems are continually lowering the cost per unit, making mass pharmaceutical production more economically viable.
A critical technical focus is the selection and engineering of the biodegradable matrix material. Hyaluronic acid (HA) is a frontrunner due to its biocompatibility, rapid dissolution kinetics, and ability to encapsulate sensitive biological molecules without compromising their activity. Researchers are focusing on modifying HA and other polymer backbones (like PLGA) to tune the dissolution profile—ranging from immediate release (ideal for vaccines) to sustained release over several days (suitable for chronic disease therapies). This kinetic control is achieved through careful cross-linking, composite structuring, and layering techniques within the microneedle body, enabling multi-drug delivery or optimized therapeutic profiles.
Furthermore, the development of integrated applicator systems is crucial for ensuring reliable clinical performance and patient acceptance. These systems, often proprietary to key market players, guarantee consistent force application, ensuring all microneedles penetrate the stratum corneum barrier effectively, independent of user technique. The next wave of innovation focuses on 'smart' microneedle patches incorporating micro-sensors or wireless connectivity. These patches track skin temperature or local biomarkers before and after application, providing data points crucial for personalized dosing and confirming successful drug delivery, thereby blurring the line between diagnostic and therapeutic technologies and driving the high valuation of integrated system patents.
Regional Highlights
- North America (United States and Canada): North America maintains its position as the dominant market shareholder, driven by substantial venture capital funding channeled into specialized biotech firms developing microneedle platforms. The U.S. FDA’s increasing acceptance of combination products and the presence of major pharmaceutical innovators (e.g., 3M, BD, Kindeva) facilitate high levels of clinical trial activity and early commercialization of complex biologic delivery systems. The region's high healthcare expenditure and willingness to adopt premium, innovative drug delivery systems, particularly in chronic disease management (such as obesity and diabetes care), fuel consistent revenue growth. Government initiatives focusing on pandemic preparedness have also accelerated investment in microneedle-based thermostable vaccine platforms, reinforcing regional leadership in the innovation pipeline.
- Europe (Germany, UK, France, Italy, Spain): Europe represents the second-largest market, characterized by stringent regulatory standards (EMA) but strong government support for innovative research, particularly in the UK and Germany. The focus in Europe is heavily tilted toward dermatology, cosmetics, and advanced wound healing applications initially, though major pharmaceutical collaborations are now driving growth in systemic therapeutics. Adoption is gradually increasing due to growing awareness of the benefits of self-administration, especially within aging populations managing long-term conditions. The market is highly influenced by specialized academic institutions and CDMOs specializing in high-precision microfabrication, partnering with regional pharmaceutical heavyweights to optimize manufacturing processes and secure market entry across multiple member states.
- Asia Pacific (APAC) (China, Japan, South Korea, India): The APAC region is poised for the highest CAGR throughout the forecast period. This rapid expansion is underpinned by vast, underserved patient populations, rapid improvements in healthcare infrastructure, and burgeoning domestic biotechnology industries, particularly in South Korea and China. South Korea is a leader in cosmetic and dermatological microneedle products, benefiting from favorable regulatory pathways and consumer acceptance. Japan, with its strong emphasis on quality and precision manufacturing, is a key hub for technology refinement. Additionally, the need for stable, easily deployable vaccines in large populations across India and Southeast Asia makes dissolvable microneedles a highly attractive logistical solution for governmental immunization programs, attracting large-scale investments in local manufacturing capabilities.
- Latin America (LATAM): The LATAM market is in an nascent stage but demonstrates significant long-term potential, primarily driven by the need for cost-effective, easily accessible drug delivery solutions. Market penetration is currently limited by economic volatility and slower regulatory processes compared to North America or Europe. However, government and non-governmental organization (NGO) procurement of vaccine patches for public health campaigns is expected to be a key entry point for the technology. Brazil and Mexico are leading regional adoption due to their substantial urban populations and relatively advanced pharmaceutical distribution networks, focusing initially on pain management and general therapeutic applications that prioritize convenience and compliance.
- Middle East & Africa (MEA): The MEA market is the smallest but strategically important, particularly for highly thermostable vaccine delivery in remote or rural areas lacking robust cold chain infrastructure. The Gulf Cooperation Council (GCC) countries, driven by high per capita healthcare spending and investment in medical tourism and personalized medicine, are adopting high-end cosmetic and specialty therapeutic microneedle products. However, the broader African continent’s adoption will be dictated primarily by international aid and public health initiatives focused on infectious disease control, where the logistical benefits of microneedles offer a transformative advantage over traditional injectable systems.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Dissolvable Microneedle Transdermal Drug Delivery Technology Market.- 3M Company
- Vaxxas Pty Ltd.
- Zosano Pharma Corporation (Acquired by Assertio Holdings)
- Becton, Dickinson and Company (BD)
- Micropoint Technologies Inc.
- Corium International Inc.
- Nanopass Technologies Ltd.
- LTS Lohmann Therapie-Systeme AG
- Nitto Denko Corporation
- Kindeva Drug Delivery, Inc.
- Cosmed Pharmaceutical Co., Ltd.
- MicroTrans Transdermal Technologies
- MyLife Technologies B.V.
- Debiotech SA
- PharmaTher Inc.
- Verndari Inc.
- Takeda Pharmaceutical Company Limited
- Merck KGaA
- Cipla Inc.
- Small Pharma Inc.
Frequently Asked Questions
Analyze common user questions about the Dissolvable Microneedle Transdermal Drug Delivery Technology market and generate a concise list of summarized FAQs reflecting key topics and concerns.What are the primary advantages of dissolvable microneedles over traditional hypodermic injections?
Dissolvable microneedles offer superior patient compliance by eliminating needle phobia, are minimally invasive and painless, and often enhance drug stability by allowing encapsulation in a solid, thermostable matrix. They also reduce the risk of needle-stick injuries and are ideal for self-administration, improving accessibility for chronic care and vaccination programs outside clinical settings.
Which therapeutic areas are leading the adoption of dissolvable microneedle technology?
The leading therapeutic areas are Vaccine Delivery, specifically for influenza and emerging infectious diseases due to improved logistical efficiency, and Endocrinology, particularly for systemic delivery of biologics like insulin and growth hormone, where frequent, convenient, and painless self-administration is necessary for long-term patient adherence.
What are the key manufacturing challenges hindering the mass commercialization of these patches?
Major manufacturing challenges include achieving high-volume production scalability while maintaining GMP compliance, ensuring perfect uniformity in needle geometry and drug loading across every unit, and reducing the high capital expenditure required for specialized micro-molding or drawing equipment necessary for commercial-scale throughput.
How does the material composition affect the performance of dissolvable microneedle arrays?
The material, typically hyaluronic acid (HA) or biodegradable polymers like PLGA, dictates the dissolution rate and drug encapsulation efficiency. HA offers rapid dissolution for immediate release applications (like vaccines), while more complex polymers allow for tunable dissolution kinetics, enabling controlled or sustained release profiles crucial for long-acting therapeutic agents.
How is regulatory uncertainty impacting market growth for microneedle drug delivery systems?
Regulatory uncertainty, particularly surrounding the classification of microneedle patches as drug-device combination products, creates complex and lengthy approval pathways (e.g., navigating both FDA/EMA device and drug divisions). Harmonization and clear, unified regulatory guidance are essential market enablers that would accelerate clinical trials and reduce commercialization risks for pharmaceutical partners.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
