Electroporators Sales Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 432396 | Date : Dec, 2025 | Pages : 246 | Region : Global | Publisher : MRU
Electroporators Sales Market Size
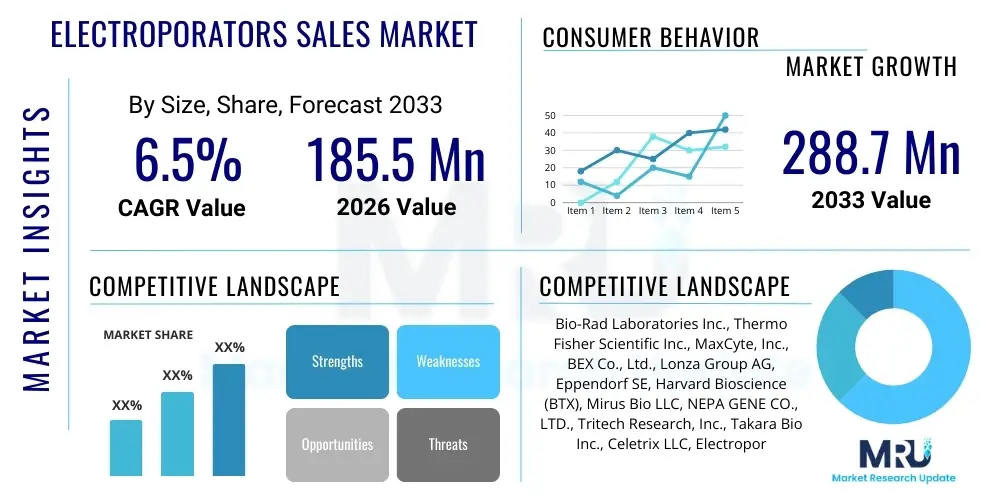
The Electroporators Sales Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.5% between 2026 and 2033. The market is estimated at $185.5 Million in 2026 and is projected to reach $288.7 Million by the end of the forecast period in 2033.
Electroporators Sales Market introduction
The Electroporators Sales Market encompasses the manufacturing, distribution, and utilization of sophisticated devices designed to transiently increase the permeability of cell membranes using high-voltage electrical pulses. This process, known as electroporation or electropermeabilization, is fundamentally critical for introducing molecules, such as DNA, RNA, proteins, and drugs, into target cells that would otherwise be impermeable. These instruments are vital tools across diverse fields, fundamentally supporting advancements in genetic engineering, synthetic biology, and therapeutic development. The core product categories include sophisticated cuvette-based systems, versatile plate-based high-throughput platforms, and specialized in vivo electroporators used for clinical and preclinical applications, particularly in vaccine development and gene therapy protocols. These devices offer a non-viral, efficient, and relatively safe method for molecular transfer, positioning them as essential equipment in modern biotechnology research labs, driving widespread adoption across global academic and industrial sectors.
Major applications driving market demand span fundamental biological research, drug discovery and development, and advanced clinical applications. In research settings, electroporation is indispensable for generating stable cell lines, cloning, and performing transient transfection experiments with high reliability. Therapeutically, the technology is leveraged heavily in gene therapy manufacturing, specifically in the production of CAR T-cells and other engineered immune cells, where precise and high-viability nucleic acid delivery is paramount for clinical success. Furthermore, in vivo electroporation systems are increasingly employed in electrochemotherapy for localized cancer treatment, offering a synergistic approach when combined with certain chemotherapeutic agents, and for enhancing the efficacy of DNA vaccines by improving cellular uptake and subsequent antigen presentation. The versatility and efficiency of these systems in handling various cell types, including historically hard-to-transfect primary cells and induced pluripotent stem cells, significantly contribute to their widespread adoption across academic, pharmaceutical, and specialized biotechnology sectors globally, necessitating continuous innovation in device design and pulse technology.
The market expansion is substantially driven by the rapid growth of the biopharmaceutical industry, particularly the accelerating pipeline of cell and gene therapies requiring robust and scalable cell engineering tools. Technological benefits, such as high efficiency, reproducibility, and minimal cell toxicity compared to chemical methods, cement electroporation’s role as the gold standard for many molecular delivery applications, especially where genetic modification must be performed under cGMP conditions. Key driving factors include increasing government and private sector funding for genomics and proteomics research, the rising prevalence of chronic diseases necessitating novel therapeutic approaches, and continuous innovations in electroporation pulse technology, leading to benchtop systems that are easier to use, offer better throughput, and require less extensive protocol optimization. The crucial integration of automation features and compatibility with large-scale biomanufacturing processes are pivotal in transitioning electroporation from a niche research tool to a critical, industrialized component of commercial therapeutic production workflows worldwide, further accelerating market trajectory.
Electroporators Sales Market Executive Summary
The Electroporators Sales Market is experiencing robust growth driven primarily by structural shifts within the biopharmaceutical sector towards personalized medicine and advanced therapeutics, notably gene and cell therapies. Business trends indicate a strong emphasis on developing closed, automated electroporation systems compatible with Current Good Manufacturing Practices (cGMP), facilitating seamless scale-up from research to clinical production. Strategic collaborations between electroporator manufacturers and Contract Development and Manufacturing Organizations (CDMOs) specializing in viral vector and cell therapy production are defining the competitive landscape, aiming to integrate technology offerings for optimized workflow efficiency, compliance, and reduced turnaround times. Furthermore, manufacturers are focusing intensely on developing pulsed-field generators capable of delivering diverse and highly controlled waveforms (e.g., square wave, exponential decay, multiphasic pulses) suitable for specialized and sensitive cell lines, enhancing overall application flexibility and performance metrics, thereby capturing specialized niches within the academic and industrial research communities globally. This innovation focus is crucial for maintaining competitive advantage in a market increasingly demanding precision, minimal cell damage, and superior scalability for billion-cell processing runs.
Regionally, North America maintains market dominance due to high investment in biotech R&D, the presence of major pharmaceutical companies, and favorable regulatory environments supporting rapid clinical trials involving advanced therapeutics. This region is characterized by early adoption of the highest-end automated systems for commercial cell therapy production. However, the Asia Pacific (APAC) region is projected to exhibit the highest CAGR, propelled by expanding biotechnology infrastructure in countries like China, India, and South Korea, coupled with significant governmental initiatives and investment to foster local life science research and biomanufacturing capabilities. European countries, particularly Germany and the UK, remain vital hubs for academic research and novel therapeutic development, focusing heavily on adopting high-throughput and robotic electroporation systems to accelerate drug screening processes and support emerging European cell therapy clinical programs. The globalization of clinical trial activities and the establishment of decentralized cell therapy manufacturing sites worldwide are influencing regional sales dynamics, leading to increased demand for portable, robust, and validated electroporation equipment that can meet stringent global standards and harmonized regulatory requirements.
Segmentation trends highlight the rapid expansion of the Reagent-Free Electroporation segment, which is increasingly preferred for its reduced operational complexity, elimination of potential reagent toxicity, and diminished regulatory burden in clinical applications, though traditional Cuve-based Systems maintain a strong foothold in academic basic research due to their simplicity, lower initial cost, and extensive legacy protocol base. Application-wise, the Cell and Gene Therapy segment is the dominant revenue generator and fastest-growing category, reflecting the therapeutic breakthroughs in oncology and rare diseases utilizing engineered T-cells and hematopoietic stem cells. Product segmentation analysis shows that high-throughput plate-based electroporators are capturing significant market share in industrial settings (e.g., vaccine production, high-content screening, and rapid protocol optimization), while dedicated In Vivo Electroporation devices are witnessing increased uptake in preclinical research and certain clinical procedures like electrochemotherapy, necessitating precision, minimal invasiveness, and high localized efficacy. Ultimately, the market trajectory is defined by the symbiotic relationship between technological innovation, stringent regulatory compliance, and the expansive clinical adoption of genetically modified therapeutic cells.
AI Impact Analysis on Electroporators Sales Market
User inquiries regarding the impact of Artificial Intelligence (AI) on the Electroporators Sales Market often revolve around three core themes: optimizing transfection parameters, enhancing experimental throughput and data interpretation, and integrating AI into automated cGMP manufacturing lines. Users are keenly interested in whether AI algorithms can predict the optimal voltage, pulse length, and buffer composition necessary for maximum transfection efficiency and cell viability for novel or difficult-to-transfect cell lines, thereby significantly reducing extensive, time-consuming, and resource-intensive manual optimization studies that currently characterize the field. Concerns also focus on how AI-driven image analysis can automatically monitor cell health post-electroporation, identify compromised cells, and automate quality control in high-throughput screens, ensuring batch consistency. The overarching expectation is that AI and Machine Learning (ML) will transform electroporation from an inherently empirical, trial-and-error technique into a predictive science, accelerating research timelines, reducing reagent waste, and ensuring superior batch-to-batch consistency in critical large-scale therapeutic manufacturing workflows, thereby reducing manufacturing failure rates and operational costs.
- AI algorithms optimize complex electroporation parameters (voltage, pulse width, multi-pulse sequencing, buffer composition, and cell density) based on historical cellular metadata, reducing experimental variability and optimization time by up to 70%.
- Integration of Machine Learning (ML) into high-throughput systems enables predictive modeling of cell viability and transfection success rates for novel cell lines, enhancing operational efficiency and resource allocation during preclinical testing.
- AI-driven image analysis facilitates automated post-electroporation quality control (QC) by precisely counting transfected cells, assessing membrane integrity, and quantifying stress markers, ensuring real-time monitoring of batch quality.
- Predictive maintenance analytics, powered by AI, are used to monitor electroporator hardware performance, track the lifespan of key components, and predict potential failures, thereby minimizing expensive and critical downtime in regulatory-sensitive cGMP manufacturing environments.
- AI assists in standardizing data reporting, protocol validation, and cross-platform comparability by autonomously normalizing data sets, which is crucial for accelerating regulatory submission processes related to personalized cell and gene therapies manufactured using advanced electroporation systems.
DRO & Impact Forces Of Electroporators Sales Market
The Electroporators Sales Market is significantly shaped by powerful drivers, substantial restraints, and burgeoning opportunities that collectively determine its growth trajectory over the forecast period. The primary driver is the exponentially increasing global demand for commercially viable cell and gene therapies, which rely heavily on efficient and non-viral genetic modification methods for therapeutic cell manufacturing, coupled with the consistent governmental and private funding influx into life sciences, functional genomics, and personalized medicine research worldwide. However, the market faces notable restraints, primarily the high initial capital investment required for purchasing advanced, automated high-throughput electroporation systems, which can be prohibitive for smaller research centers, alongside the significant technical complexity involved in optimizing standardized protocols for sensitive primary cells, posing a barrier to universal adoption. Opportunities are vast, particularly in the rapid expansion of clinical applications like non-invasive tissue treatment and the development of next-generation solid-state pulsed power technologies offering enhanced portability, superior control, and lower energy consumption. These market forces interact dynamically, with the critical clinical demand acting as a magnetic pull overcoming cost restraints, while relentless technological advancements continually unlock new therapeutic and research potentials, particularly through the use of microfluidics and integrated automation.
Market Drivers
The core engine for market growth stems from the paradigm shift towards advanced therapeutic modalities. The regulatory approval and successful commercialization of breakthrough therapeutic products, such as autologous CAR T-cell therapies, have solidified electroporation’s status as a critical, validated upstream processing step in clinical manufacturing, ensuring that the technology is now indispensable for both late-stage clinical trials and commercial-scale operations globally. This heightened clinical relevance drives manufacturers to innovate rapidly, focusing on developing closed, cGMP-compliant systems that meet stringent global regulatory requirements for patient safety and product quality in therapeutic production. This foundational demand is non-cyclical and tied directly to the robust pipeline of oncology and rare disease treatments, guaranteeing long-term market stability and growth.
A secondary, yet crucial, driver is the continuous technological refinement within the device segment itself, significantly improving user experience and biological outcomes. Newer generations of electroporators feature superior and highly refined control over pulse parameters (voltage kinetics, duration, number and spacing of pulses), optimized electrode and chamber designs minimizing cell loss and overheating, and integrated flow-through capabilities suitable for continuous processing environments. These technical improvements translate directly into higher cell viability and dramatically improved transfection efficiency, successfully addressing historical challenges associated with severe cell death during the electroporation procedure. Additionally, the increasing trend toward laboratory automation and industrial robotics in bioprocessing facilities mandates the adoption of plate-based and automated pipetting systems that seamlessly incorporate electroporation, inherently boosting sales of complex, integrated high-end units capable of processing large cell quantities quickly and reliably, thereby accelerating drug discovery pipelines significantly and increasing reliance on consumables.
Market Restraints
Despite strong underlying demand, the market growth is moderately constrained by the significant capital expenditure required for sophisticated, high-end, cGMP-compliant electroporation equipment, often exceeding $100,000 to $200,000 for specialized clinical-grade systems with flow capabilities. This substantial upfront cost barrier significantly limits adoption, particularly in developing economies, mid-sized academic laboratories, or smaller biotechnology startups operating under restricted budgetary allocations and tight grant funding cycles. Compounding this financial hurdle is the persistent technical sensitivity and variability of the electroporation process itself; protocol optimization remains highly cell-type dependent and challenging, requiring specialized technical expertise and extensive validation. Inconsistent results across different labs or even different batches within the same facility due to minor variations in cell handling, buffer composition, or pulse application can lead to serious reproducibility issues, raising concerns among researchers and regulatory bodies and occasionally leading to the preference for established, albeit sometimes less efficient, viral transduction methods when feasible and clinically appropriate.
Furthermore, concerns regarding inherent cytotoxicity and cellular stress induced by the high-voltage electrical pulses present a physical restraint to universal adoption, especially for fragile or rare cell populations like hematopoietic stem cells. While recent advancements have successfully reduced cell mortality, the unavoidable stress inflicted on highly sensitive primary cells or fragile engineered cell types remains a drawback, sometimes necessitating extensive, expensive, and time-consuming downstream recovery protocols that increase total process time and cost. Another regulatory restraint emerges in clinical applications: the requirement for strict validation, traceability, and batch record keeping of every single electroporation parameter within a therapeutic manufacturing process adds substantial complexity and time to the approval pathway and ongoing operations. The fragmented landscape of proprietary buffer formulations and high-value disposable consumables associated with specific electroporator models further complicates procurement, inventory management, and standardization efforts, contributing to operational complexity and vendor lock-in for global multi-site organizations aiming for uniform protocols and centralized purchasing strategies.
Market Opportunities
Significant market opportunities are emerging from the technological overlap between molecular delivery and novel clinical needs, particularly in gene editing. The rapid development of non-viral gene editing tools, such as CRISPR/Cas systems, which often rely on delivering large, highly charged ribonucleoprotein (RNP) complexes into cells, inherently requires highly efficient and low-toxicity delivery mechanisms. This positions electroporation as the primary delivery vehicle for RNPs into various cell types, representing a substantial, untapped market for optimized RNP-specific electroporation buffers, custom protocols, and dedicated systems. Furthermore, the development of localized, non-invasive in vivo electroporation technologies for targeted drug and gene delivery to specific internal organs, solid tumors, or localized tissue types is opening entirely new therapeutic avenues beyond traditional ex vivo cell modification, promising reduced systemic toxicity, improved patient compliance, and superior efficacy in fields like oncology, cardiology, and dermatology.
Moreover, the burgeoning global market for personalized medicine, including patient-specific neoantigen cancer vaccines and highly tailored autologous cell therapies, necessitates localized, small-batch, rapid manufacturing capabilities close to the point of care. This decentralized manufacturing trend creates immense demand for compact, semi-automated, highly reliable, and easily scalable benchtop electroporators suitable for deployment in hospital-based manufacturing hubs or specialized clinical laboratories. From a technological perspective, robust opportunities exist in integrating advanced microfluidic technologies with electroporation, enabling extremely precise, ultra-high-throughput, and low-volume processing capabilities, significantly reducing expensive reagent consumption and minimizing cellular mechanical stress due to superior fluid dynamics control. The necessary shift towards proprietary, high-value disposable consumables (e.g., specialized electroporation plates and cuvettes with integrated sensors) designed for specific, high-stakes therapeutic applications represents a key revenue stream opportunity for market leaders to secure long-term, high-margin recurring income streams and enhance valuable customer retention rates through consumable optimization.
Segmentation Analysis
The Electroporators Sales Market is comprehensively segmented based on the product type, application area, end-user profile, and specific technology utilized in pulse generation and delivery. This segmentation provides a granular view of market dynamics, highlighting areas of accelerated growth, established revenue streams, and emerging niche markets. Product segmentation differentiates between the durable capital equipment (instruments) and the recurring necessity of consumables (cuvettes, plates, buffers, reagents), with the latter providing a continuous and resilient revenue base due to their disposable nature and high volume utilization in clinical settings. Application analysis centers heavily on cell and gene therapy due to its profound and sustained growth rate, contrasting with foundational research uses in academic labs. End-user analysis distinguishes between highly regulated and high-volume pharmaceutical and biotechnology companies requiring cGMP compliance and budget-constrained, but numerically superior, academic and research institutions, while technology differentiates specialized pulsed-field systems based on high-throughput capacity versus standard batch processing, enabling highly targeted strategic sales and marketing efforts across diverse client bases and budgetary constraints.
- By Product Type: Instruments (Benchtop Electroporators, Flow-based Systems, In Vivo Devices), Consumables (Electroporation Cuvettes, Multi-Well Plates, Proprietary Buffers & Reagents, Electrodes).
- By Application: Cell and Gene Therapy Manufacturing (CAR T-cells, Stem Cells), Drug Discovery and Development (Target Validation, High-Content Screening), Academic Research (Genomics, Proteomics), Cancer Research (Electrochemotherapy), Vaccine Development (DNA Vaccines, Nucleic Acid Delivery).
- By Technology: Traditional Electroporation (Cuvette-based/Batch Processing), High-Throughput Electroporation (Plate-based, Automated Systems), In Vivo Electroporation (Needle/Surface Electrode Systems), Microfluidic Electroporation.
- By End User: Pharmaceutical and Biotechnology Companies, Academic and Research Institutions (Universities, Government Labs), Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs), Hospitals and Clinical Laboratories.
Value Chain Analysis For Electroporators Sales Market
The intricate value chain supporting the Electroporators Sales Market necessitates a high degree of specialization and coordination among various sophisticated stakeholders, starting from the specialized electronic component suppliers. Upstream complexity is rooted in the requirement for extremely precise, high-voltage, and highly reliable power delivery systems, which are crucially governed by proprietary waveform algorithms developed by the main equipment manufacturers. This necessitates long-term strategic agreements with niche semiconductor and advanced electrical engineering firms capable of meeting rigorous specifications for component durability, accuracy, and ultra-fast switching capabilities necessary for pulse control. Furthermore, the profitable consumables segment, involving sterile cuvettes, proprietary buffer components, and specialty polymer plates, relies heavily on chemical and polymer manufacturers who must adhere to stringent medical-grade standards, ensuring minimal leaching or contamination that could potentially compromise sensitive biological samples or high-value therapeutic cell lines. Intellectual property protection and trade secrets around these buffer chemistries and precise pulse sequences are a key source of competitive advantage at the upstream procurement and manufacturing level, securing strong differentiation and maintaining high gross margins on recurring consumable sales.
The core manufacturing stage involves the precision assembly, rigorous calibration, and extensive functional testing of the electroporation instruments. Manufacturers in this specialized space invest heavily in R&D to refine the system user interface, integrate automated liquid handling robotics, and ensure the systems are seamlessly compatible with existing laboratory informatics management systems (LIMS) and data traceability protocols, thus optimizing workflow integration and compliance for end-users. Regulatory compliance is profoundly paramount here; systems destined for clinical or cGMP manufacturing environments must pass extensive validation protocols, often including IQ/OQ/PQ documentation and requiring stringent 21 CFR Part 11 compliance for electronic record keeping and data integrity, significantly increasing manufacturing overheads. Midstream logistics are substantially challenged by the sensitive nature of the equipment, requiring specialized shipping, precise climate control, and expert handling to prevent damage to internal calibration components and high-voltage circuitry. This manufacturing and compliance complexity drives up both production costs and the final price point, contributing directly to the capital cost restraint factor observed in the overall market analysis.
Downstream market penetration is achieved through meticulously managed, dual distribution channels tailored to specific customer profiles. Direct sales forces are indispensable for engaging major pharmaceutical, established biotechnology, and specialized clinical clients, providing personalized technical consultations, offering multi-year comprehensive service contracts, and delivering the highly complex application training necessary for clinical implementation and validation. These direct channels facilitate the crucial technical dialogue required for customized solutions, such as integrating electroporators into large, closed, automated cell processing isolators or bespoke clinical facilities. In stark contrast, the indirect channel, leveraging global scientific equipment distributors and regional dealers, plays a vital role in reaching thousands of smaller academic labs and emerging biotech entities worldwide. The indirect model emphasizes efficient inventory management, rapid standard delivery, and competitive pricing, particularly for entry-level standard benchtop models and commodity consumables. The overall effectiveness of the value chain ultimately hinges on the manufacturer's ability to successfully balance these two divergent distribution strategies while providing consistent, high-quality post-sales technical support and complete regulatory documentation essential for maintaining client trust and securing a long-term, dominant market presence across all geographical regions.
Electroporators Sales Market Potential Customers
The primary purchasers and key end-users of electroporation systems are institutions engaged in high-stakes, cutting-edge life science research, comprehensive drug development, and regulated therapeutic manufacturing. Pharmaceutical and established biotechnology companies represent the highest-value customer segment, driven by their critical, non-negotiable need for robust, scalable, and cGMP-compliant systems essential for manufacturing high-value cell and gene therapy products, including CAR T-cells, TCR therapies, and proprietary engineered cell lines utilized in large-scale drug screening assays. These strategic customers prioritize superior performance metrics such as automation, extremely high throughput capability, minimal cell toxicity, and verifiable, consistent reproducibility, often leading to significant, large-volume purchases of integrated plate-based systems and associated proprietary consumables, creating essential long-term, high-margin revenue streams for system vendors.
Academic and governmental research institutions (including NIH-funded labs and national laboratories) constitute the largest volume of purchasers globally, driven primarily by competitive grants and foundational research needs in functional genomics, systems biology, and fundamental cell biology. While these high-volume buyers often opt for simpler, more affordable cuvette-based or smaller benchtop systems due to budgetary constraints and lower throughput needs, their continuous and dispersed need for standard consumables ensures a steady, essential market base for all vendors. Furthermore, the rapidly rising number of Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs) globally, specifically those specializing in preclinical testing, translational research, and therapeutic cell production, are rapidly emerging as significant potential customers. CROs/CMOs require flexible, multi-purpose, and rapidly reconfigurable electroporators capable of efficiently handling diverse client projects, placing a premium on system versatility, rapid protocol development, and comprehensive validation support, thereby driving substantial demand for high-specification, flexible instruments that can rapidly switch between different applications, cell types, and regulatory requirements while maximizing capital efficiency and throughput.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | $185.5 Million |
| Market Forecast in 2033 | $288.7 Million |
| Growth Rate | 6.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Bio-Rad Laboratories Inc., Thermo Fisher Scientific Inc., MaxCyte, Inc., BEX Co., Ltd., Lonza Group AG, Eppendorf SE, Harvard Bioscience (BTX), Mirus Bio LLC, NEPA GENE CO., LTD., Tritech Research, Inc., Takara Bio Inc., Celetrix LLC, Electroporation Technologies LLC, INOVIO Pharmaceuticals Inc., Biocept, Inc. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Electroporators Sales Market Key Technology Landscape
The technology landscape in the Electroporators Sales Market is characterized by continuous refinement aimed aggressively at increasing transfection efficiency while simultaneously minimizing cell death and stress, thereby effectively addressing the core trade-off inherent in the electroporation technique. The primary technical differentiation among competing instruments lies in the precise, proprietary control over electrical pulse delivery, moving strategically from simple, legacy square-wave pulses to highly complex, user-defined, or proprietary multi-phasic waveforms that are optimally designed to maximize reversible pore formation and molecular uptake specifically for challenging cell types (e.g., primary immune cells, induced pluripotent stem cells). Modern, industrial-grade systems leverage sophisticated solid-state power supplies, advanced real-time monitoring sensors, and specialized microprocessors to deliver highly repeatable and reproducible pulse sequences, which is an absolute necessity for all clinical and commercial industrial applications where batch consistency is regulatory mandated.
Two critical technological trends are overwhelmingly dominating the current landscape: high-throughput automation systems and advanced flow-based electroporation techniques. High-throughput electroporators utilize multi-well plates (e.g., 96- or 384-well formats) and are seamlessly integrated with automated liquid handling robotics and plate readers to process hundreds or thousands of unique samples rapidly, making them indispensable tools for comprehensive drug screening, critical target validation, and large-scale manufacturing quality control checks. This technology significantly minimizes the manual labor and time required for high-volume, repetitive tasks prevalent in large pharmaceutical and biotech R&D departments. Concurrently, flow-based or continuous-flow electroporation systems are rapidly gaining traction as the standard in large-scale therapeutic production. These advanced devices allow billions of cells suspended in a liquid medium to pass through a highly controlled electrical field continuously and efficiently, enabling scalable processing of massive cell volumes per run, effectively bridging the critical gap between small bench-scale experimentation and demanding commercial therapeutic production requirements, offering superior scalability and consistency compared to static batch systems.
Another profound area of intense technological development is the integration of high-precision microfluidics and significant miniaturization. Microfluidic electroporation utilizes precisely fabricated micro-channels and chips to control cell movement and electrical exposure at the highly granular, single-cell level or across small, controlled cell populations, offering extremely high transfection efficiency with minimal cellular stress due to highly precise control over localized field strength, temperature dissipation, and fluid shear forces. While currently predominantly utilized in specialized, high-resolution research and diagnostic assays, microfluidic systems promise to reduce expensive reagent consumption significantly and offer unparalleled physical control, paving the way for next-generation portable and advanced point-of-care devices for rapid clinical diagnostics and personalized therapy manufacturing. Furthermore, ongoing research into specialized non-contact electrode designs and novel electroporation methods (e.g., leveraging localized optical fields or controlled transient cavitation) continues, aiming vigorously to improve application safety, enhance efficacy, and reduce off-target effects, particularly in sensitive in vivo applications where precision targeting and minimal surrounding tissue damage are paramount for achieving optimal therapeutic outcomes and minimizing adverse patient reactions.
Regional Highlights
Regional dynamics are critical in defining the global Electroporators Sales Market, reflecting profound variations in localized R&D investment patterns, the maturity of regulatory frameworks governing cell therapies, and established biomanufacturing capacities across different continents. North America, particularly the United States, holds the commanding global market share, underpinned by the largest concentration of leading global pharmaceutical and biotechnology firms, robust and sustained funding mechanisms (NIH, substantial venture capital), and a highly mature ecosystem for complex clinical trials focusing on advanced cell and gene therapies. The region is characterized by early and high adoption rates of premium, fully automated, and validated systems necessary for large-scale, continuous bioproduction. Europe follows as the second-largest market, driven by strong foundational academic research in countries like Germany, the UK, and France, coupled with significant governmental support for advanced medical research and sophisticated biotechnology infrastructure development, prioritizing instruments that comply strictly with stringent EU safety and quality standards (e.g., CE marking and relevant directives).
- North America: Market leader and primary revenue generator due to pioneering advancements in clinical cell and gene therapy, the highest concentration of global biopharma headquarters, and leading R&D expenditure. The strategic focus is intensely concentrated on procuring cGMP-compliant, high-throughput, and fully automated systems for commercial therapeutic manufacturing and late-stage clinical trials.
- Asia Pacific (APAC): Recognized as the fastest-growing and most dynamic regional market, fueled by rapid and sustained expansion of biotechnology infrastructure, substantial governmental investments in local life sciences clusters (especially in China, Japan, and India), and a rapidly increasing number of local CMOs/CDMOs supporting both regional and global therapeutic production needs. Demand is rapidly polarizing for both affordable, reliable academic systems and high-end, validated industrial systems.
- Europe: A highly mature and established market characterized by robust public research funding mechanisms (e.g., Horizon Europe) and strong, historical adoption in specialized electrochemotherapy applications for cancer treatment. Countries like Germany, Switzerland, and the UK are key markets for advanced research electroporators, academic genomics studies, and integrated laboratory automation solutions requiring high precision and validated performance.
- Latin America (LATAM) & Middle East & Africa (MEA): Emerging markets showing moderate but steady growth, primarily driven by increasing healthcare modernization efforts, expansion of university research budgets, and the establishment of initial biotech research centers focused on infectious diseases and local agriculture. Demand is highly focused on essential, cost-effective laboratory equipment, relying more heavily on efficient indirect distribution channels and focusing on reliable benchtop units for academic research and smaller clinical labs.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Electroporators Sales Market.- Bio-Rad Laboratories Inc.
- Thermo Fisher Scientific Inc.
- MaxCyte, Inc.
- BEX Co., Ltd.
- Lonza Group AG
- Eppendorf SE
- Harvard Bioscience (BTX)
- Mirus Bio LLC
- NEPA GENE CO., LTD.
- Tritech Research, Inc.
- Takara Bio Inc.
- Celetrix LLC
- Electroporation Technologies LLC
- INOVIO Pharmaceuticals Inc.
- Biocept, Inc.
Frequently Asked Questions
Analyze common user questions about the Electroporators Sales market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary factor driving the demand for Electroporators in the current market?
The primary factor is the global boom in Cell and Gene Therapy (CGT) manufacturing. Electroporation provides a highly efficient, validated, non-viral method for delivering large nucleic acids (DNA/RNA, RNP) into sensitive T-cells and stem cells, making it indispensable for the commercial production of FDA-approved and clinical pipeline CGT products requiring robust cGMP-compliant transfection solutions and high cell viability.
How do high-throughput electroporators differ fundamentally from traditional cuvette-based systems?
High-throughput electroporators utilize automated, scalable plate-based systems (e.g., 96 or 384 wells) compatible with industrial robotics, allowing the simultaneous, rapid processing of thousands of samples for drug screening, protocol optimization, or large-scale manufacturing quality control. Traditional cuvette-based systems are simpler, less capital-intensive, and designed for small-batch processing in basic academic research settings.
What are the key technical challenges currently facing Electroporation technology adoption?
The key technical challenges include minimizing inherent cytotoxicity (cell death) caused by the high-voltage electrical pulse, optimizing complex and specific protocols for highly sensitive primary human cell lines, and developing cost-effective, scalable closed systems that can handle the massive cell volumes required for commercial therapeutic production while rigorously maintaining stringent sterility and regulatory compliance standards across global sites.
Which geographical region is expected to exhibit the fastest revenue growth in the Electroporators Market over the forecast period?
The Asia Pacific (APAC) region is strongly forecasted to experience the fastest Compound Annual Growth Rate (CAGR). This accelerated growth is primarily driven by substantial governmental and private sector investments in localized biotechnology parks, the rapidly increasing volume of pharmaceutical R&D activities, and rising domestic manufacturing capabilities for advanced therapeutics in emerging markets like China, South Korea, and India.
What is the strategic role of Artificial Intelligence (AI) in advancing next-generation Electroporation systems?
AI is strategically integrated to autonomously optimize complex pulse parameters (voltage kinetics, waveform shape, pulse duration) based on cellular metadata, thereby maximizing transfection efficiency and post-procedure cell viability while significantly reducing manual optimization time. AI also enhances quality control by analyzing post-electroporation cell images and providing crucial predictive maintenance alerts for manufacturing equipment.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
