Angiogenesis Assay Kit Market Size, By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 443190 | Date : Feb, 2026 | Pages : 258 | Region : Global | Publisher : MRU
Angiogenesis Assay Kit Market Size
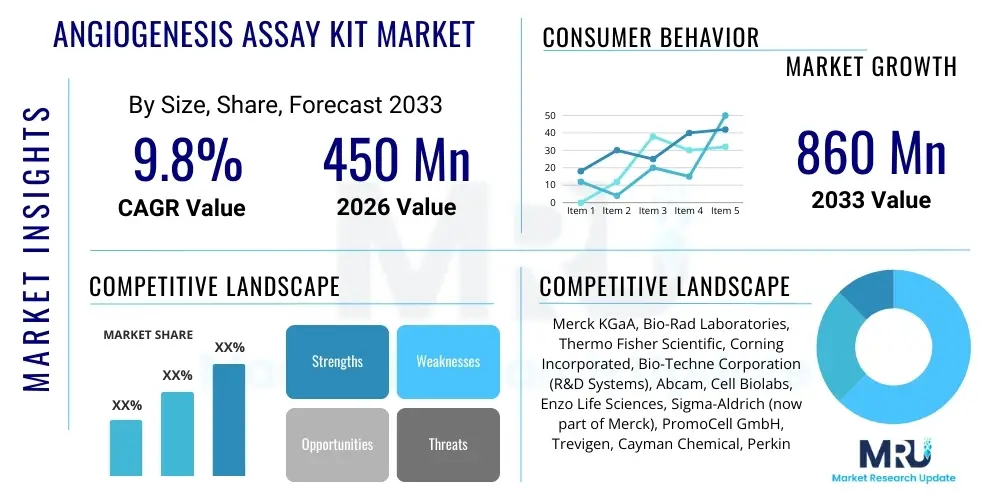
The Angiogenesis Assay Kit Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 9.5% between 2026 and 2033. The market is estimated at USD 450 Million in 2026 and is projected to reach USD 860 Million by the end of the forecast period in 2033.
Angiogenesis Assay Kit Market introduction
The Angiogenesis Assay Kit Market comprises specialized laboratory tools and reagents designed to study the complex process of angiogenesis—the formation of new blood vessels from pre-existing vasculature. These kits are indispensable in biological and pharmacological research, particularly in oncology, cardiovascular disease research, wound healing studies, and ophthalmology. Angiogenesis is a critical biological pathway implicated in both physiological processes (like development and tissue repair) and pathological conditions (such as tumor growth and metastatic progression). The development of novel therapeutics targeting this process relies heavily on accurate, reproducible, and high-throughput assays provided by these kits. The market offers a variety of assay formats, including cell-based assays (tube formation, migration, invasion), biochemical assays (ELISA, activity assays), and ex vivo models (aortic ring assays), allowing researchers to select the most relevant system for their specific research goals.
Major applications of these assay kits span the entire drug discovery pipeline, from initial target validation and screening of potential anti-angiogenic or pro-angiogenic compounds to understanding the mechanisms of drug resistance. The inherent complexity and variability of biological systems necessitate standardized, reliable kits, driving market demand. Key benefits derived from using commercial kits include reduced experimental setup time, enhanced standardization compared to in-house protocols, improved reproducibility across different labs, and higher sensitivity and throughput necessary for large-scale screening initiatives. The availability of kits optimized for various cell types and species further broadens their utility across diverse research environments.
Driving factors for market expansion include the significant global increase in cancer research funding, as aberrant angiogenesis is a hallmark of nearly all solid tumors. Furthermore, the rising prevalence of chronic diseases, such as age-related macular degeneration (AMD) and diabetic retinopathy, which are characterized by abnormal neovascularization, fuels demand for sophisticated assay systems. Technological advancements, such as the integration of 3D cell culture techniques and microfluidics into assay formats, are enhancing the physiological relevance of these assays, attracting pharmaceutical and biotechnology companies seeking improved preclinical models for efficacy testing. This technological push, coupled with increasing investments in personalized medicine, ensures sustained market growth.
Angiogenesis Assay Kit Market Executive Summary
The Angiogenesis Assay Kit Market is experiencing robust growth driven by accelerating investments in oncology and chronic disease therapeutics globally. Business trends highlight a shift toward physiologically relevant models, particularly 3D cell culture and organ-on-a-chip technologies integrated into assay platforms, offering higher predictive capabilities compared to traditional 2D systems. Major pharmaceutical and biotechnology companies are increasingly outsourcing or adopting commercial kits to enhance the efficiency and scalability of their drug screening processes. Furthermore, strategic collaborations between kit manufacturers and academic institutions are common, aiming to validate new assay technologies and expand application protocols. Competitive dynamics are focused on kit performance metrics, including speed, precision, and the ability to measure multiple endpoints simultaneously, promoting rapid innovation in assay design and reagent formulation.
Regionally, North America maintains the largest market share due to substantial governmental and private sector funding for biomedical research, the strong presence of leading pharmaceutical and biotech firms, and early adoption of advanced laboratory technologies. However, the Asia Pacific region is projected to exhibit the fastest growth rate, fueled by improving research infrastructure in countries like China, India, and South Korea, coupled with increasing prevalence of chronic diseases and expanding regulatory support for clinical trials and drug development. Europe remains a significant market, characterized by stringent quality standards and a strong focus on basic biological mechanism research, contributing steadily to global market revenue.
Segmentation trends indicate that cell-based assays, particularly tube formation and migration assays, continue to dominate the market owing to their high relevance in simulating in vivo processes. However, biochemical assays are gaining traction due to their high throughput capabilities, essential for primary drug screening. By end-user, pharmaceutical and biotechnology companies constitute the primary revenue generators, driven by large-scale compound screening requirements, while academic and research institutes represent a stable, foundational customer base focused on mechanistic studies. The trend toward standardized, multiplexed kits that can measure several angiogenic factors simultaneously is defining product development across all key segments.
AI Impact Analysis on Angiogenesis Assay Kit Market
Common user questions regarding AI's impact on the Angiogenesis Assay Kit Market revolve primarily around improving data interpretation, automating image analysis, and predicting assay outcomes. Users are keen to understand how machine learning can handle the vast amounts of complex, high-dimensional data generated by modern high-content screening (HCS) angiography assays, particularly in quantifying tube formation morphology and vascular network complexity. Concerns often center on the validation and generalizability of AI algorithms trained on diverse cell lines and experimental conditions, alongside the necessary investment in sophisticated instrumentation and computational infrastructure required for implementation. Expectations are high regarding AI's ability to streamline the drug discovery pipeline by rapidly identifying active compounds and reducing false positives, thereby enhancing the efficiency and cost-effectiveness of preclinical angiogenic testing.
AI is transforming the interpretation phase of angiogenesis assays, moving beyond manual or semi-automated counting of tubes and junctions. Advanced deep learning models are capable of analyzing complex microscopic images from assays (such as HUVEC tube formation) to extract subtle quantitative morphological metrics that correlate more accurately with in vivo efficacy. This capability significantly reduces observer bias and speeds up the analysis, allowing researchers to evaluate compound libraries much faster. Furthermore, AI platforms can integrate assay output data with genomic, proteomic, and clinical data to build predictive models, helping researchers prioritize candidates and design more effective follow-up experiments.
The integration of AI, particularly in high-content imaging platforms, increases the throughput potential of angiogenesis assays dramatically. Automated image segmentation, feature extraction, and classification powered by convolutional neural networks (CNNs) standardize the measurement of parameters like total tube length, branching points, and average mesh size across thousands of wells. This automation facilitates robust quality control and allows for rapid dosage optimization and toxicity screening early in the drug development process. Consequently, the value proposition of highly standardized and instrument-compatible assay kits is augmented, driving innovation toward formats optimized for automated robotic handling and AI-driven analysis pipelines.
- AI optimizes high-content image analysis by automating morphological quantification.
- Machine learning models improve data interpretation efficiency and reduce observer variability in tube formation assays.
- Predictive AI algorithms correlate assay results with downstream clinical efficacy markers.
- Integration of AI facilitates high-throughput screening, accelerating drug candidate identification.
- AI aids in quality control by detecting and flagging anomalies in complex assay data sets.
- Development of proprietary algorithms enhances the value of advanced, standardized assay kits.
DRO & Impact Forces Of Angiogenesis Assay Kit Market
The Angiogenesis Assay Kit Market is propelled by increasing research funding directed toward oncology and chronic inflammatory diseases (Drivers). However, the complexity of biological models and the need for expensive high-content screening infrastructure act as significant restraints (Restraints). Opportunities lie in the development of in vivo-mimicking 3D models and the expansion into niche applications like regenerative medicine (Opportunities). These factors collectively generate an impact force that mandates innovation in assay sophistication and cost-effectiveness to meet the high demands of the pharmaceutical industry and bridge the gap between in vitro results and clinical outcomes.
Drivers fueling the market include the rising global incidence of cancer, necessitating continuous investigation into novel anti-angiogenic therapies and the underlying mechanisms of tumor vascularization. Increased government funding and private venture capital investment in drug discovery and personalized medicine platforms directly translate into higher demand for robust, standardized assay systems. Furthermore, technological advancements leading to the development of kits that mimic physiological conditions more accurately, such as those incorporating extracellular matrix components and multi-cellular co-culture systems, attract researchers seeking high-fidelity preclinical data. The necessity for quick and repeatable results in high-throughput screening environments also mandates the use of reliable commercial kits.
Restraints primarily encompass the inherent biological complexity and variability associated with angiogenesis research, making it challenging to develop universal assay systems that perfectly predict in vivo responses. The high cost associated with advanced assay kits, particularly those designed for 3D culture and high-content analysis, can limit adoption in smaller academic laboratories or budget-constrained organizations. Additionally, regulatory hurdles and the extended time required for the validation of new assay technologies before widespread commercial adoption pose limitations. The lack of complete standardization across different manufacturers and assay formats sometimes complicates the comparison and reproducibility of results across various research groups.
Opportunities for growth are significant in the integration of microfluidics (lab-on-a-chip) technology with angiogenesis assays, creating dynamic platforms that better simulate blood flow and mechanical stimuli found in vivo. The emerging field of regenerative medicine and tissue engineering presents a new market segment where pro-angiogenic kits are required to optimize vascularization for tissue grafts and artificial organs. Furthermore, expanding geographical reach into emerging markets in the APAC region, coupled with focused efforts to develop cost-effective, medium-throughput kits suitable for smaller labs, will unlock new revenue streams. The continuous drive towards personalized medicine also offers opportunities for specialized assays that test patient-specific vascular responses.
Segmentation Analysis
The Angiogenesis Assay Kit Market is comprehensively segmented based on Assay Type, Application, End-User, and geographical region. This segmentation provides a granular understanding of consumer preferences and technological trends. Assay type segmentation distinguishes between highly quantitative, often automated biochemical assays and physiologically relevant, complex cell-based assays. Application analysis highlights the dominant role of oncology research, while also capturing significant contributions from cardiovascular and ophthalmological studies. End-user categorization reflects the primary market drivers—pharmaceutical and biotechnology companies—and the foundational research carried out by academic and research institutes.
Analyzing these segments reveals critical shifts in market dynamics. The increasing sophistication of drug discovery efforts favors kits that offer high fidelity and throughput, driving demand within the pharmaceutical sector for standardized, validated protocols. Geographic segmentation underscores the maturity of the North American and European markets, coupled with the rapid expansion potential within the Asia Pacific. Furthermore, the convergence of various assay formats into multiplex kits that can measure migration, proliferation, and tube formation simultaneously is a key trend across all applications, offering researchers comprehensive data sets from a single experiment and thus optimizing resources.
- By Assay Type:
- Cell-Based Assays (Tube Formation Assays, Migration/Invasion Assays, Proliferation Assays, Sprouting Assays)
- Biochemical Assays (ELISA, Western Blotting, Activity Assays)
- Ex Vivo Assays (Aortic Ring Assays, Chick Embryo Assays)
- By Application:
- Oncology Research
- Drug Discovery and Development
- Cardiovascular Disease Research
- Ophthalmology (AMD, Diabetic Retinopathy)
- Wound Healing/Tissue Engineering
- By End-User:
- Pharmaceutical and Biotechnology Companies
- Academic and Research Institutes
- Contract Research Organizations (CROs)
- Diagnostic Laboratories
- By Region:
- North America (U.S., Canada)
- Europe (Germany, U.K., France, Italy, Spain, Rest of Europe)
- Asia Pacific (Japan, China, India, South Korea, Rest of APAC)
- Latin America (Brazil, Mexico, Rest of Latin America)
- Middle East and Africa (South Africa, GCC Countries, Rest of MEA)
Value Chain Analysis For Angiogenesis Assay Kit Market
The value chain for the Angiogenesis Assay Kit Market begins with upstream raw material suppliers, predominantly focusing on specialized biochemicals, growth factors, cell culture media components, and sophisticated imaging reagents. Quality control and sourcing reliability at this initial stage are paramount, as the performance and reproducibility of the final kit depend heavily on the purity and consistency of these inputs. Key activities in the upstream phase involve the purification and formulation of highly specific antibodies, recombinant proteins, and proprietary matrices used in various assay formats. Strategic partnerships with key component suppliers are crucial for manufacturers to maintain competitive pricing and ensure continuous supply of specialized, high-grade biological materials.
The core manufacturing stage involves the assembly, quality validation, and packaging of the complete kits, often requiring cleanroom facilities and adherence to strict manufacturing protocols. Distribution channels represent the critical link to the end-users. This involves both direct sales, where large manufacturers maintain specialized sales forces to service major pharmaceutical clients and academic centers, and indirect channels, relying heavily on a global network of specialized distributors and e-commerce platforms. Distributors provide localized technical support, inventory management, and faster delivery times, particularly important for temperature-sensitive biological reagents. The efficiency of the distribution network directly influences the accessibility and market penetration of the products.
Downstream analysis focuses on the end-users—researchers in pharmaceutical companies, academia, and CROs—who utilize the kits for drug screening and mechanistic studies. The value generated downstream includes rapid identification of therapeutic candidates, cost reduction through efficient screening, and generation of high-quality scientific data. Post-sales support, including technical assistance, troubleshooting, and continuous protocol updates, is an integral part of maintaining customer loyalty and ensuring high experimental success rates. Direct feedback from downstream users is essential for iterative product improvement and the development of next-generation assay kits, completing the feedback loop in the value chain.
Angiogenesis Assay Kit Market Potential Customers
The primary consumers and end-users of Angiogenesis Assay Kits are institutions deeply involved in biomedical research and therapeutic development, driven by the need to understand and modulate vascularization processes. Pharmaceutical and biotechnology companies represent the largest and most commercially lucrative segment. These organizations routinely conduct high-throughput screening (HTS) of thousands of compounds to identify potential anti-angiogenic drugs for cancer treatment or pro-angiogenic agents for regenerative medicine. Their demand is characterized by a need for validated, standardized kits that can integrate seamlessly with automated robotic systems and high-content imaging platforms, emphasizing scale and reliability in their purchasing decisions.
Academic and governmental research institutes constitute another foundational customer base. Researchers in these environments utilize angiogenesis assays primarily for basic mechanistic studies—investigating the signaling pathways, cellular interactions, and genetic regulation underlying new blood vessel formation. While their purchasing volumes may be lower than those of industry players, their influence is critical in validating new assay methodologies and driving the adoption of innovative techniques. They prioritize experimental flexibility, physiological relevance, and robust technical support, often opting for specialized kits tailored for complex biological models like 3D co-cultures.
Contract Research Organizations (CROs) serve as a rapidly growing customer segment. Pharmaceutical and biotech companies frequently outsource preclinical toxicology and efficacy testing, including angiogenesis studies, to CROs to accelerate their pipeline development and manage costs. CROs demand high-quality, reproducible kits that can be executed quickly and reliably across various client projects. Diagnostic laboratories, although a smaller segment currently, represent future potential, especially as biomarkers related to angiogenic activity become integrated into clinical diagnostics for conditions like cancer progression and chronic inflammation.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 450 Million |
| Market Forecast in 2033 | USD 860 Million |
| Growth Rate | 9.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Merck KGaA, Bio-Rad Laboratories, Inc., Thermo Fisher Scientific, Corning Incorporated, Promega Corporation, Abcam plc, R&D Systems (a Bio-Techne brand), Cell Biolabs, Inc., AMS Biotechnology (Europe) Ltd., Angio-Proteomie, Enzo Life Sciences, Inc., QIAGEN N.V., Sgarlato Laboratories, Inc., BD Biosciences, Sigma-Aldrich (Merck), PerkinElmer Inc., FUJIFILM Wako Pure Chemical Corporation, Creative Bioarray, Boster Biological Technology, Trevigen. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Angiogenesis Assay Kit Market Key Technology Landscape
The technological landscape of the Angiogenesis Assay Kit Market is defined by continuous innovation aimed at enhancing the physiological relevance, throughput, and accuracy of vascularization studies. A major technological advancement involves the shift from traditional two-dimensional (2D) culture systems to three-dimensional (3D) models, such as spheroids, organoids, and hydrogel-based assays. These 3D systems, often incorporating complex extracellular matrices (ECMs) like Matrigel or specialized synthetic hydrogels, offer a more accurate representation of the cellular environment in vivo, leading to data with higher predictive value for clinical outcomes. Kit manufacturers are increasingly specializing in optimized reagents and protocols for these complex 3D structures, ensuring consistency and ease of use.
Another pivotal technology is the adoption of High-Content Screening (HCS) and High-Content Analysis (HCA) platforms. These automated imaging systems, coupled with specialized software for image processing and quantification, enable researchers to simultaneously measure multiple morphological and functional parameters (e.g., cell migration speed, tube formation stability, marker expression) across hundreds of wells in a single experiment. This integration of HCS capability necessitates assay kits that are fully compatible with robotic liquid handling and fluorescence microscopy, promoting the development of standardized, multi-parametric detection reagents and plates. The synergy between optimized HCS instrumentation and validated assay kits drives productivity in pharmaceutical R&D, facilitating the rapid screening of vast compound libraries.
Furthermore, microfluidics technology is emerging as a critical disruptive force. Microfluidic chips, often referred to as 'organ-on-a-chip' systems, allow for the precise control of fluid flow, shear stress, and nutrient gradients, closely mimicking the dynamic environment within blood vessels. Angiogenesis assays adapted for microfluidic platforms provide unparalleled insight into the effects of hemodynamic forces on vascular development and stability. Though currently niche, these advanced platforms are gaining traction, especially in sophisticated academic and major industry labs, because they enable longitudinal studies of vessel formation, regression, and barrier function that were previously challenging in static cultures. Kit innovation focuses on micro-channel compatible matrices and specialized cell seeding protocols to facilitate this highly precise technological application.
Regional Highlights
Geographical analysis of the Angiogenesis Assay Kit Market reveals diverse growth trajectories and adoption patterns across major regions, heavily influenced by research funding levels, regulatory environments, and the concentration of biopharmaceutical activity.
- North America: Dominates the global market, primarily due to the substantial presence of major pharmaceutical and biotechnology companies (especially in the U.S.), coupled with high government and private sector investment in cancer and cardiovascular research. The early adoption of advanced high-throughput screening technologies and a mature scientific infrastructure further solidify its leading position. The U.S. remains the largest contributor to market revenue, driven by aggressive R&D spending and numerous ongoing clinical trials related to anti-angiogenic therapies.
- Europe: Represents a significant and mature market, characterized by stringent quality standards and a strong focus on basic biological research across countries like Germany, the U.K., and France. Funding from organizations like the European Research Council (ERC) supports robust academic research, ensuring steady demand for high-quality, standardized assay kits. The market is increasingly adopting 3D culture models, reflecting a scientific preference for physiologically relevant preclinical data.
- Asia Pacific (APAC): Positioned as the fastest-growing regional market. This high growth is attributed to improving healthcare infrastructure, rising prevalence of chronic diseases, increasing governmental focus on biomedical research (particularly in China and India), and the expansion of contract research organizations (CROs). Favorable regulatory reforms and lower operational costs are attracting significant investment from global pharmaceutical companies, rapidly increasing the regional demand for research reagents and assay kits.
- Latin America & Middle East and Africa (MEA): These regions currently hold smaller market shares but offer long-term growth potential. Growth in these regions is primarily driven by expanding clinical research activities, improving access to advanced laboratory technology, and increasing international collaborations with research institutions in North America and Europe. Focus areas include basic research and disease prevalence studies specific to the regional populations.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Angiogenesis Assay Kit Market.- Merck KGaA
- Bio-Rad Laboratories, Inc.
- Thermo Fisher Scientific
- Corning Incorporated
- Promega Corporation
- Abcam plc
- R&D Systems (a Bio-Techne brand)
- Cell Biolabs, Inc.
- AMS Biotechnology (Europe) Ltd.
- Angio-Proteomie
- Enzo Life Sciences, Inc.
- QIAGEN N.V.
- Sgarlato Laboratories, Inc.
- BD Biosciences
- Sigma-Aldrich (Merck)
- PerkinElmer Inc.
- FUJIFILM Wako Pure Chemical Corporation
- Creative Bioarray
- Boster Biological Technology
- Trevigen
- MilliporeSigma
- Lonza Group AG
- Sartorius AG
- ATCC (American Type Culture Collection)
Frequently Asked Questions
Analyze common user questions about the Angiogenesis Assay Kit market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary application driving the demand for Angiogenesis Assay Kits?
The primary application driving market demand is oncology research, specifically the identification and validation of novel anti-angiogenic compounds targeting tumor vascularization, followed closely by drug discovery and development efforts for cardiovascular and ophthalmological diseases.
How are 3D cell culture systems influencing the Angiogenesis Assay Market?
3D cell culture systems are enhancing assay relevance by mimicking the physiological environment more accurately than traditional 2D models. This shift mandates the use of specialized 3D-optimized assay kits, driving innovation toward hydrogel matrices and spheroid culture protocols to provide higher predictive value for preclinical studies.
Which assay type currently dominates the Angiogenesis Assay Kit Market?
Cell-based assays, particularly the Tube Formation Assay, dominate the market due to their direct visualization of vascular structure formation, offering a high degree of biological relevance. However, biochemical assays are growing rapidly due to their high throughput capabilities necessary for early-stage drug screening.
Which geographical region is expected to show the highest growth rate?
The Asia Pacific (APAC) region is projected to exhibit the fastest Compound Annual Growth Rate (CAGR), fueled by increasing investments in life science research infrastructure, rising prevalence of chronic diseases, and the expansion of contract research organizations (CROs) in countries like China and India.
What role does Artificial Intelligence (AI) play in enhancing angiogenesis assays?
AI primarily enhances angiogenesis assays through automated high-content image analysis. Machine learning algorithms improve the efficiency and reproducibility of quantifying complex metrics (like tube length and branching points) from microscopic images, thereby accelerating high-throughput screening and reducing analysis time and observer bias.
The Angiogenesis Assay Kit Market continues its trajectory of innovation, moving toward highly complex, yet standardized, systems that offer enhanced physiological relevance. The pharmaceutical industry's persistent focus on cancer therapeutics and the escalating prevalence of chronic vascular diseases ensure sustained investment in assay technologies. The integration of cutting-edge fields like microfluidics and artificial intelligence is fundamentally reshaping how vascular biology is studied, making assays more predictive and scalable. Kit manufacturers are responding to the demand for multiplexed, high-throughput solutions that seamlessly integrate into automated laboratory environments. This technological advancement, coupled with robust research funding in North America and rapid infrastructural development in APAC, solidifies a strong growth outlook for the forecast period. Standardization and improved fidelity will remain critical competitive differentiators as the market matures and moves towards clinical relevance. Continuous technological adaptation, particularly in the realm of 3D modeling and advanced imaging software integration, is crucial for market stakeholders to maintain a leading edge and capture the expanding opportunities across diverse therapeutic areas. The demand is shifting from mere detection to functional understanding, requiring kits capable of measuring dynamic responses over time and in increasingly complex cellular environments. This market is intrinsically linked to the success of preclinical drug development, making reliability and validation paramount concerns for all end-users.
The strategic imperatives for market participants include deepening expertise in 3D culture component manufacturing and developing AI-compatible data acquisition and analysis tools. Companies must focus not only on the chemical and biological reagents but also on providing comprehensive solutions that include proprietary software and technical support tailored to advanced instrumentation. The adoption curve for microfluidic-based assays is expected to steepen, presenting a key opportunity for specialized manufacturers who can offer integrated chip-based solutions. Furthermore, expanding distribution networks into emerging markets and developing region-specific pricing strategies will be essential for capitalizing on global growth, particularly within the CRO segment which is growing rapidly globally, seeking cost-effective yet high-quality preclinical models. Long-term market stability will depend on successful translation of in vitro assay results to meaningful clinical outcomes, fostering greater trust in standardized commercial kits across the drug development ecosystem. Regulatory scrutiny, particularly concerning the validation of complex biological assays for preclinical submission, is expected to increase, further emphasizing the need for highly characterized and validated products from reputable suppliers.
In terms of specific assay formats, while the classic Tube Formation Assay (using endothelial cells on ECM matrices) remains the gold standard for quick qualitative assessment, the future belongs to highly quantitative, multi-parametric assays. These include advanced migration/invasion assays utilizing specialized chemotaxis chambers, and biochemical kits that quantify multiple angiogenic growth factors and inhibitors simultaneously. The trend towards customized assay kits that allow researchers to incorporate patient-derived cells or specific genetic modifications is also notable, catering to the burgeoning field of personalized medicine and disease modeling. This customization capability, coupled with high quality control standards, allows researchers to bridge the gap between bench research and clinical translation effectively, ensuring that compounds moving forward in the pipeline have been tested under conditions highly representative of the target disease state. This emphasis on quality and fidelity drives premium pricing for advanced kits, benefiting manufacturers who invest heavily in R&D and validation studies.
The competitive environment is characterized by large life science conglomerates offering comprehensive portfolios (e.g., Thermo Fisher Scientific, Merck KGaA) alongside specialized companies focusing on niche, high-performance assay formats (e.g., Cell Biolabs, Trevigen). Strategic acquisitions and licensing agreements are common methods for large players to integrate advanced technology from smaller innovators, ensuring continuous technological refreshment. Key areas of differentiation include the source and quality of recombinant growth factors supplied within the kits, the proprietary composition of the extracellular matrix components, and the user-friendliness of the protocols, particularly for inexperienced users. Furthermore, offering comprehensive educational resources and application notes that detail complex experimental setup (such as co-culture protocols) provides added value and supports broader market adoption of technically demanding assays. As the pharmaceutical industry leans heavily on phenotypic screening, the demand for sophisticated, reproducible functional assays like those targeting angiogenesis will only intensify, cementing the market’s positive trajectory.
Market challenges, though present, are primarily focused on the complexity of replicating in vivo environments and ensuring cost-effectiveness without compromising data quality. Addressing the restraint of high cost, manufacturers are beginning to introduce medium-throughput, modular assay kits that cater to the needs of smaller laboratories, offering flexibility without requiring full-scale HCS investment. Addressing biological complexity involves ongoing research into novel scaffolding materials and serum-free media formulations that minimize variability and mimic natural tissue environments more closely. Successfully navigating these challenges requires strong collaborations between kit providers, cell biologists, and bioengineers, ensuring that the next generation of angiogenesis assay kits are robust, affordable, and clinically predictive, thus maximizing their utility in both academic discovery and industrial development settings. The global need for new therapeutic targets for cancer and chronic inflammatory diseases ensures that the underlying research area—angiogenesis—remains a top priority, guaranteeing persistent market relevance.
The market landscape is witnessing an increasing focus on standardization protocols, particularly those driven by industry consortia and regulatory bodies. The lack of uniform assay standards historically presented difficulties in comparing results across different laboratories or even within the same lab using kits from varying sources. Leading manufacturers are actively participating in initiatives to establish benchmarks for key angiogenesis parameters, aiming to increase the confidence of drug developers in their preclinical data. This drive for harmonization is a necessary step for the broader acceptance of new assay formats, such as microfluidic chips, which offer exceptional physiological relevance but require rigorous validation against established methods. Companies that prioritize transparency in their assay validation data and provide detailed standard operating procedures (SOPs) gain a significant competitive advantage by reducing the time and cost associated with customer validation efforts. The overall trend suggests a shift towards 'solution selling,' where vendors offer integrated platforms combining kits, specialized reagents, imaging devices, and AI-driven analysis software, moving beyond simple reagent provision to comprehensive workflow optimization.
Technological advancement is not limited to 3D and AI; rapid innovations in non-invasive, real-time imaging techniques also impact kit design. Kits that are compatible with time-lapse microscopy or specialized imaging reagents (e.g., fluorescent reporters for cell migration or proliferation) allow researchers to study the kinetics of angiogenesis in unparalleled detail. This real-time analysis provides richer, dynamic data compared to end-point measurements, offering mechanistic insights crucial for understanding drug action. Furthermore, the rising interest in personalized and precision medicine necessitates assay kits that are easily adaptable to low-volume primary cell cultures, often derived from patient biopsies. This customization trend requires manufacturers to ensure their matrices and growth factor compositions are highly versatile and compatible with sensitive, patient-specific cell types, moving the market toward more bespoke, high-value offerings. The ability to model pathological vascular states (e.g., hyper-angiogenesis associated with tumors) accurately using these advanced kits remains a central theme in product development and market differentiation.
From a commercial perspective, supply chain resilience, especially following global disruptions, has become a key consideration. Assuring the timely and reliable delivery of temperature-sensitive biological components is paramount. Manufacturers are investing in more robust cold chain logistics and diversifying their sourcing to mitigate future risks, ensuring that essential research activities are not hampered by delays. Pricing strategies are complex, balancing the high R&D cost associated with innovative 3D and microfluidic-compatible kits against the competitive pressure from generic biochemical assay manufacturers. Companies successfully deploying a tiered pricing strategy—offering basic, cost-effective kits for high-throughput screening and premium, high-fidelity kits for mechanistic validation—are achieving balanced market penetration. The continuous cycle of pharmaceutical innovation means the demand for sophisticated preclinical tools will persist, reinforcing the long-term viability and growth prospects of the Angiogenesis Assay Kit Market across all key end-user segments globally.
Investment in customer education and technical support is becoming increasingly critical, particularly as assays become more biologically complex. Researchers transitioning from basic 2D models to sophisticated 3D co-cultures often require significant assistance with cell handling, matrix preparation, and complex imaging protocols. Leading vendors are providing extensive online resources, webinars, and dedicated field application specialists to ensure high customer success rates. This commitment to educational outreach not only builds brand loyalty but also accelerates the adoption of advanced assay technologies. Successful market penetration in regions like APAC also relies heavily on localized technical support in native languages, addressing regional scientific community needs effectively. The interplay between product quality and support infrastructure is a defining feature of the competitive strategy in this niche, yet scientifically critical, market segment.
Moreover, the ethical considerations surrounding animal testing are indirectly boosting the demand for high-fidelity in vitro and ex vivo models, including advanced angiogenesis assay kits. As regulatory pressure increases to reduce or replace animal use in preclinical testing, researchers are actively seeking alternative methods that provide equivalent or superior predictive power. Advanced human cell-based angiogenesis assays, particularly those incorporating multiple cell types (e.g., endothelial cells, pericytes, and tumor cells) and housed within microfluidic systems, are emerging as viable and often preferred alternatives to traditional animal models. This regulatory and ethical push provides a significant long-term growth driver for the developers of sophisticated, animal-free assay kits, aligning market growth with broader societal and scientific objectives regarding research methodology improvement. This secular trend ensures continuous evolution and optimization of assay technology.
Finally, the convergence of genomics and proteomics with functional angiogenesis assays is creating opportunities for highly specialized kits. Researchers are increasingly integrating genetic knockdown/knockout studies with functional assays to pinpoint specific molecular targets affecting vascular development. Assays that facilitate easy collection of media for subsequent proteomic analysis (e.g., cytokine profiling using multiplex ELISA) or allow for easy cell lysis for downstream genomic analysis are highly valued. This demand for integrated solutions that bridge molecular biology with functional phenotyping drives the development of multi-modal kits, catering to the most cutting-edge research questions in oncology and regenerative medicine. The development of kits that seamlessly integrate into standardized laboratory information management systems (LIMS) is also essential for maintaining data integrity and traceability in high-throughput environments, particularly within large pharmaceutical R&D departments and clinical research organizations.
The regulatory environment, particularly in North America and Europe, plays a crucial role in shaping product development. Manufacturers must ensure their kits meet high standards of consistency and reliability, especially those intended for use in GLP/GCP-compliant preclinical safety studies. Documentation, batch-to-batch consistency, and robust quality management systems are non-negotiable requirements, acting as natural barriers to entry for smaller or less established players. The ability to provide comprehensive Certificate of Analysis (CoA) and validation reports for every kit batch is a key differentiating factor. This regulatory necessity further emphasizes the market preference for established, large-scale manufacturers with proven track records in quality control and supply chain management within the life science tools sector. Investment in regulatory compliance is thus a critical component of market strategy for long-term sustainability and credibility.
Looking ahead, the next phase of innovation in the Angiogenesis Assay Kit Market is expected to focus heavily on automation and standardization of 3D modeling. This involves developing pre-plated, ready-to-use 3D hydrogel matrices and standardization of cell sourcing to minimize user variability. Furthermore, there is a clear trend toward integrating physiological parameters, such as oxygen tension and pH control, directly into the assay kits or associated devices to create even more realistic cellular environments. The ongoing battle against cancer, where the understanding and manipulation of tumor vasculature are central to therapeutic success, will continue to fuel the demand for the most predictive and technologically advanced angiogenesis assessment tools available on the market, sustaining the strong growth rate projected through 2033. The continuous collaboration between technology providers and the research community remains the cornerstone of innovation in this vital sector of the life science industry.
The rise of customized and patient-derived cell models underscores a major commercial pivot. Rather than solely offering generic human umbilical vein endothelial cells (HUVECs) assays, manufacturers are developing protocols and specialized media components optimized for induced pluripotent stem cell (iPSC)-derived endothelial cells (iECs). iECs provide a pathway for generating patient-specific vascular models, which is essential for pharmacogenomics and personalized drug testing. Kits that facilitate the efficient differentiation and maintenance of iECs for subsequent angiogenesis assays command premium pricing and capture high-value contracts with biopharmaceutical companies focused on precision medicine initiatives. This specialization increases the complexity of the kits but delivers unparalleled biological relevance, making the upfront investment justified by the potential reduction in failure rates during later clinical trial phases.
The competitive rivalry in the market is often centered on proprietary matrix formulations. Extracellular matrix hydrogels, such as those based on animal-derived proteins (e.g., Matrigel) or synthetic polymers, are core components of many functional assays. Companies continually strive to improve the stability, transparency (for imaging), and handling properties of these matrices. Developing synthetic, chemically defined matrices that eliminate batch-to-batch variability and regulatory concerns associated with animal products is a major focus area for R&D spending. Successful deployment of a superior, fully defined synthetic matrix could significantly disrupt the market dominance of traditional animal-derived products, providing a cleaner, more standardized foundation for reproducible angiogenesis research globally. This technological race among manufacturers directly benefits researchers by offering increasingly reliable and defined experimental systems.
Finally, the market must address the global need for simplified, rapid assays suitable for point-of-care diagnostics or rapid screening in low-resource settings, particularly in the context of infectious disease research or emergency wound care where swift assessment of vascular status might be beneficial. While the majority of current kits are geared toward complex laboratory research, the development of simpler, lateral flow or micro-spot assays targeting key angiogenic biomarkers (like VEGF or Ang-2) presents an untapped niche opportunity. Successfully translating the complexity of angiogenesis assessment into a user-friendly, rapid format could unlock significant new market segments beyond the traditional R&D laboratories, further expanding the application base and overall revenue potential of the Angiogenesis Assay Kit Market in the coming years. This diversification of product offerings will be key to capturing the full spectrum of biomedical demands.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
