Durezol Market Size, By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 441650 | Date : Feb, 2026 | Pages : 255 | Region : Global | Publisher : MRU
Durezol Market Size
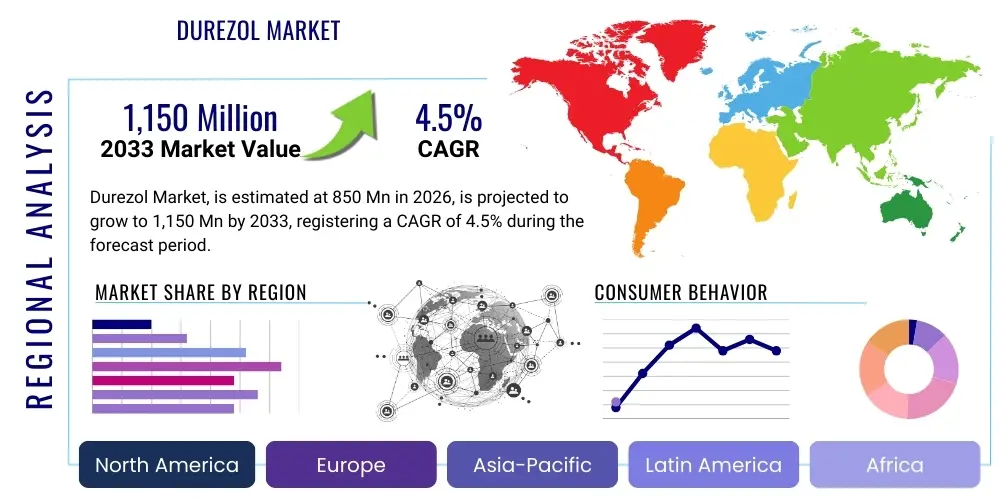
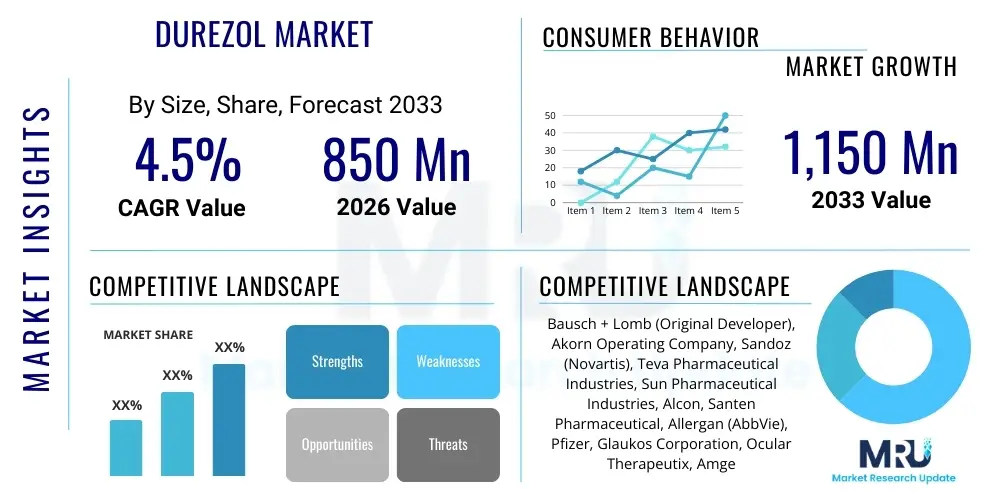
The Durezol Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 4.5% between 2026 and 2033. The market is estimated at $850 Million USD in 2026 and is projected to reach $1,150 Million USD by the end of the forecast period in 2033. This growth trajectory is primarily supported by the increasing prevalence of ophthalmic surgeries, particularly cataract removal, coupled with Durezol's established efficacy profile in managing post-operative inflammation and pain. Despite facing increasing generic competition in certain regions, the high potency and unique formulation benefits of Durezol (difluprednate ophthalmic emulsion) continue to secure its position as a preferred steroid option among ophthalmologists, especially for complex or high-risk surgical cases.
Durezol Market introduction
The Durezol market encompasses the sales and distribution of difluprednate ophthalmic emulsion, a highly potent topical corticosteroid indicated for the treatment of inflammation and pain associated with ocular surgery, notably cataract surgery. Durezol, renowned for its strong anti-inflammatory action, offers the advantage of less frequent dosing compared to traditional corticosteroids, typically administered twice daily, enhancing patient compliance and therapeutic outcomes. This product is a crucial component of post-operative care regimens, designed to minimize complications such as corneal edema, breakdown of the blood-aqueous barrier, and potential vision loss, thereby driving its essential role in the ophthalmic pharmaceutical landscape.
Major applications of Durezol extend beyond routine cataract surgery to include the management of endogenous anterior uveitis and other inflammatory conditions affecting the anterior segment of the eye. The pharmaceutical’s oil-in-water emulsion formulation allows for superior ocular surface retention and bioavailability, ensuring effective penetration of the drug into ocular tissues. This enhanced therapeutic delivery is a key differentiator in a competitive market segment characterized by other steroidal and non-steroidal anti-inflammatory drugs (NSAIDs). The primary benefits include rapid resolution of inflammation, significant pain reduction, and a minimized dosing schedule which improves the quality of life for patients recovering from invasive procedures.
Driving factors for sustained market expansion include the global demographic shift toward an aging population, which inherently increases the incidence of age-related eye diseases requiring surgical intervention. Furthermore, advancements in surgical techniques, such as micro-incisional cataract surgery (MICS), mandate effective and rapid control of post-operative inflammation to achieve optimal visual rehabilitation. Regulatory acceptance and inclusion in major clinical guidelines further solidify Durezol’s market penetration. However, pricing pressure from biosimilars and generics, coupled with managed care scrutiny over specialty drug costs, necessitates continuous market adaptation and demonstration of superior clinical value by the brand manufacturer.
Durezol Market Executive Summary
The Durezol market is characterized by stable demand driven by demographic factors, offset partially by intense pricing competition stemming from generic entry in key geographic regions. Business trends indicate a focus on optimizing manufacturing efficiency and extending market reach into emerging economies where cataract surgical volumes are rapidly increasing but access to advanced post-operative care is expanding. Key stakeholders are leveraging clinical data demonstrating Durezol’s superior therapeutic index to maintain premium pricing relative to older generation steroids and newly introduced generics, emphasizing reduced treatment duration and overall efficacy. Strategic partnerships with ambulatory surgical centers (ASCs) and large ophthalmology practices are central to market share retention, especially in highly competitive North American and European markets.
Regional trends highlight North America as the dominant revenue generator, supported by high healthcare expenditure, established reimbursement pathways, and a large volume of elective ophthalmic surgeries. Conversely, the Asia Pacific (APAC) region is projected to exhibit the highest growth rate due to improvements in healthcare infrastructure, increased disposable incomes leading to greater adoption of advanced treatments, and massive unmet needs in surgical ophthalmology. Europe maintains a steady trajectory, heavily influenced by national health service procurement policies and a stronger push toward cost-effective generic alternatives. Successfully navigating the varied regulatory and reimbursement landscapes across these regions requires localized market entry strategies focusing on either volume expansion or value-based differentiation.
Segment trends reveal that the Application segment dominated by post-operative inflammation treatment continues to be the primary revenue stream. Within the Distribution Channel segment, hospital pharmacies and specialty clinics maintain prominence due to the prescription-only nature and specialization required for administering Durezol. Future growth is anticipated in formulations offering even greater convenience, potentially including drug delivery systems that extend the dosing interval further or offer combination therapy with antibiotics. The overall market dynamics suggest a maturing product lifecycle, requiring innovation not necessarily in the active ingredient itself, but in formulation science and patient adherence programs to secure long-term market vitality against biosimilar erosion.
AI Impact Analysis on Durezol Market
Common user questions regarding AI's impact on the Durezol market primarily center on how artificial intelligence can optimize ophthalmic treatment protocols, specifically the management of post-operative inflammation. Users frequently inquire about AI’s role in predicting patient response to corticosteroids like Durezol, determining personalized dosing schedules to minimize side effects (like intraocular pressure spikes), and automating the diagnostic assessment of inflammation severity post-surgery. There is significant interest in AI applications that could integrate data from Electronic Health Records (EHRs) and imaging diagnostics to flag patients at higher risk of complications, ensuring timely and appropriate intervention with high-potency drugs like Durezol, thereby moving treatment away from generalized protocols toward precision medicine in ophthalmology.
The integration of AI tools is expected to significantly enhance the clinical decision-making process for prescribing specialized ophthalmic drugs. Specifically, machine learning algorithms can analyze vast datasets of patient characteristics, surgical history, and inflammatory markers to create predictive models that gauge the likelihood of an individual requiring a potent anti-inflammatory regimen such as Durezol. This enhanced precision reduces unnecessary drug exposure for low-risk patients while ensuring high-risk individuals receive optimal treatment promptly. For the Durezol manufacturer, this translates into AI supporting evidence-based marketing, focusing on segments where the drug offers the greatest differentiated clinical benefit based on predictive outcomes.
Furthermore, AI-driven applications could revolutionize clinical trials and post-market surveillance. Natural Language Processing (NLP) and machine vision could accelerate the analysis of patient-reported outcomes (PROs) and ophthalmic imaging (e.g., anterior segment OCT, slit-lamp photography) used to measure inflammation resolution. This rapid data processing allows manufacturers to swiftly gather real-world evidence confirming Durezol's effectiveness and safety profile in diverse patient populations. Over the long term, AI may influence the development of next-generation topical corticosteroids by identifying novel delivery mechanisms or combination therapies that overcome current limitations, potentially shaping future competitive strategies in the anterior segment drug market.
- AI-enhanced Diagnostic Precision: Machine learning algorithms aid in grading post-operative inflammation (e.g., flare and cells), optimizing the timing and duration of Durezol treatment.
- Personalized Dosing Regimens: AI models predict individual patient response to difluprednate, minimizing the risk of adverse events like steroid-induced ocular hypertension.
- Clinical Trial Optimization: AI accelerates data analysis from Phase III and IV trials, improving recruitment and endpoint assessment efficiency for novel ophthalmic formulations.
- Supply Chain Management: Predictive analytics driven by AI models forecast regional demand fluctuations based on surgical trends and disease outbreaks, ensuring efficient distribution.
- Adherence Monitoring: AI-powered digital health tools track patient compliance with the prescribed twice-daily Durezol regimen, linking adherence to clinical outcomes.
DRO & Impact Forces Of Durezol Market
The Durezol market is dynamically shaped by powerful forces of demographic change, surgical innovation, stringent regulatory requirements, and competitive pressure from generic alternatives. Drivers are primarily concentrated around the globally expanding elderly population, which requires a constantly increasing volume of cataract and refractive surgeries, creating a sustained demand for effective post-operative management solutions. Furthermore, ophthalmologists’ preference for high-potency, low-dosing corticosteroids due to better patient compliance and superior efficacy often steers prescription patterns toward Durezol. Restraints include the market entry of lower-cost generic versions of difluprednate, which exert significant downward pressure on pricing, especially in countries with centralized purchasing systems. Additionally, the potential for steroid-related side effects, such as increased intraocular pressure (IOP), necessitates careful monitoring and limits the use of the drug in certain high-risk patient subgroups, presenting a clinical constraint.
Opportunities for growth are heavily focused on geographical expansion into rapidly developing economies in Asia, Latin America, and the Middle East, where modernization of healthcare infrastructure and rising affluence are making specialized ophthalmic care more accessible. Development of sustained-release drug delivery systems, such as implants or punctal plugs loaded with difluprednate, represents a significant technological opportunity. Such innovations would eliminate the need for daily drops, revolutionizing patient adherence and providing enhanced therapeutic consistency, thereby offering a clear competitive advantage over existing topical drops, including generic versions. Furthermore, the exploration of combination therapies, integrating difluprednate with suitable antibiotics or NSAIDs, could capture a broader market share by addressing multifaceted post-operative needs in a single product.
Impact forces govern the overall market momentum. The intensity of rivalry is exceptionally high due to the presence of multiple branded and generic competitors in the topical ophthalmic corticosteroid space. Substitute threat is moderate to high, with NSAIDs often used as alternatives, although they generally lack the potent anti-inflammatory effect of steroids like Durezol. Regulatory scrutiny, particularly concerning the long-term safety data and manufacturing quality, serves as a crucial barrier to entry, favoring established players. The shifting power of buyers, represented by large purchasing groups and insurance payers, is a persistent challenge, demanding robust pharmacoeconomic data to justify the product's premium cost. Therefore, sustained growth relies heavily on proving superior patient outcomes and offsetting the impact of genericization through formulation innovation and targeted clinical marketing.
Segmentation Analysis
The Durezol market is comprehensively segmented based on its application, distribution channel, and geographic region, reflecting the diverse clinical needs and commercial pathways for this specialized pharmaceutical product. Segmentation by application is critical, differentiating between primary uses such as post-operative ocular inflammation and pain (the dominant segment) and the treatment of specific inflammatory diseases like endogenous anterior uveitis. Understanding these application subsets allows manufacturers to tailor marketing materials and clinical studies to specific practitioner groups, demonstrating the drug’s specialized benefits where standard treatments may fall short.
Segmentation by Distribution Channel reflects the specialized nature of ophthalmic prescribing. Due to the requirement for specific medical oversight regarding steroid use and potential IOP complications, Durezol is typically dispensed through hospital pharmacies, specialty pharmacies, and retail pharmacies with high-volume prescription capacity. The shift toward Ambulatory Surgical Centers (ASCs) for cataract surgeries globally also creates a distinct sub-segment, as ASCs often maintain streamlined inventory systems and bulk purchasing agreements, making them key strategic partners for market penetration and volume stability.
Geographic segmentation is vital for resource allocation and regulatory strategy. North America and Europe command the largest market value due to advanced healthcare systems and established market access. Conversely, Asia Pacific and Latin America offer high growth potential, driven by expanding surgical capacity and increasing public awareness of advanced eye care treatments. Analyzing these segments ensures that pricing, reimbursement strategies, and educational initiatives are customized to meet the local socioeconomic and regulatory environment, optimizing overall commercial performance and maximizing patient access.
- Application:
- Post-Operative Ocular Inflammation and Pain (Primary segment)
- Endogenous Anterior Uveitis
- Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Specialty Pharmacies
- Ambulatory Surgical Centers (ASCs)
- Region:
- North America
- Europe
- Asia Pacific (APAC)
- Latin America (LATAM)
- Middle East & Africa (MEA)
Value Chain Analysis For Durezol Market
The value chain for Durezol begins with extensive pharmaceutical research and development, focusing on the formulation science necessary to stabilize difluprednate in an emulsion suitable for ophthalmic use, ensuring high bioavailability and patient comfort. The upstream activities involve the sourcing and synthesis of the active pharmaceutical ingredient (API) and specialized excipients, requiring adherence to stringent Good Manufacturing Practices (GMP). Efficient API production and quality control are critical since the specialized nature of the emulsion formulation dictates precise manufacturing protocols. Strategic partnerships with key raw material suppliers are essential to mitigate supply chain risks and ensure cost-effective procurement, a significant factor given the competitive pressure in the final drug market.
The midstream process includes manufacturing, packaging, and regulatory approval. Manufacturing involves sterile filling and sophisticated quality assurance to guarantee the integrity of the ophthalmic emulsion. Following production, distribution forms the critical link between the manufacturer and the end-user. Distribution channels are highly regulated, primarily utilizing specialized pharmaceutical distributors who manage temperature-controlled logistics to hospitals, specialty clinics, and retail pharmacies. Direct channels (e.g., direct-to-hospital sales teams) are often employed for high-volume accounts and governmental procurement, while indirect distribution relies on wholesale networks to reach smaller prescribing centers and retail outlets, ensuring broad market coverage across varied geographic areas.
Downstream activities center on prescription and patient use. This involves intensive marketing and medical education efforts targeting ophthalmologists, optometrists, and surgical staff to ensure appropriate prescribing practices and patient education regarding the twice-daily dosing regimen and potential side effects. The final stage involves the payer market, where complex negotiations with insurance providers and Pharmacy Benefit Managers (PBMs) determine coverage and reimbursement levels, significantly impacting patient access and overall market success. The effectiveness of the product in real-world settings, documented through post-marketing studies, continuously feeds back into the value chain, driving continuous refinement in manufacturing processes and clinical communication strategies to sustain market positioning.
Durezol Market Potential Customers
The primary consumers and prescribing influencers of Durezol are specialized medical professionals and institutions focused on anterior segment eye care. The largest segment of end-users comprises ophthalmologists, particularly those specializing in cataract, corneal, and refractive surgery, as Durezol is predominantly prescribed for the management of post-operative pain and inflammation. These surgeons prioritize high-efficacy treatments that reduce recovery time and minimize the risk of complications, aligning perfectly with Durezol’s profile as a potent steroid with a favorable dosing schedule. Institutional buyers, such as hospitals and Ambulatory Surgical Centers (ASCs), are also crucial customers, as they purchase the drug in bulk for use in their surgical procedure kits and inpatient/outpatient formularies, driven by considerations of cost-effectiveness, stock availability, and clinical guidelines established by their medical committees.
A secondary, yet important, customer segment includes optometrists who co-manage surgical patients and may initiate the prescription or refill process, especially in regions where their scope of practice permits therapeutic prescribing. Additionally, patients suffering from non-surgical inflammatory conditions like acute anterior uveitis represent another key segment. While smaller in volume than the post-operative market, this segment requires sustained high-potency anti-inflammatory treatment, where Durezol’s therapeutic strength offers distinct advantages over less potent alternatives. The satisfaction and adherence of these individual patients, guided by their prescribing physician, ultimately drive repeat prescription volume and brand loyalty, even in the face of generic alternatives.
Ultimately, the key purchasing decision is heavily influenced by large institutional payers and government health systems (in regions like Europe and Canada), which determine whether Durezol is included on preferred drug lists (formularies) and at what level of co-pay. Therefore, successful market penetration requires demonstrating clear pharmacoeconomic value—proving that the superior efficacy and reduced dosing associated with Durezol lead to better long-term outcomes and reduced overall healthcare costs (e.g., fewer re-admissions or complications) compared to generic or alternative treatments. Targeting PBMs and managed care organizations with robust health economics and outcomes research (HEOR) data is essential to unlock sustained access and sales volume.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | $850 Million USD |
| Market Forecast in 2033 | $1,150 Million USD |
| Growth Rate | 4.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Bausch + Lomb (Original Developer), Akorn Operating Company, Sandoz (Novartis), Teva Pharmaceutical Industries, Sun Pharmaceutical Industries, Alcon, Santen Pharmaceutical, Allergan (AbbVie), Pfizer, Glaukos Corporation, Ocular Therapeutix, Amgen, Bristol-Myers Squibb, Johnson & Johnson, Cipla. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Durezol Market Key Technology Landscape
The technological landscape surrounding the Durezol market is primarily defined by advanced ophthalmic drug delivery systems aimed at enhancing efficacy, improving bioavailability, and extending drug residence time on the ocular surface. Durezol itself is based on sophisticated emulsion technology—specifically, an oil-in-water emulsion. This formulation is crucial as it allows the lipophilic difluprednate molecule to be efficiently delivered to the ocular surface, promoting excellent penetration into the anterior segment tissues (cornea and aqueous humor). The emulsion structure minimizes irritation and often requires lower total drug concentration to achieve therapeutic effect compared to older suspension formulations, thereby supporting the twice-daily dosing frequency which is a key competitive advantage in patient compliance.
Beyond the core emulsion technology, emerging advancements focus heavily on sustained-release drug delivery platforms. These technologies aim to move away from traditional eye drops entirely, addressing the significant issue of patient non-adherence and inconsistent dosing. Key technological innovations include drug-eluting punctal plugs, which are inserted into the tear drainage system to slowly release difluprednate over several weeks post-surgery, providing constant therapeutic levels. Similarly, bio-erodible ocular inserts and subconjunctival injectable depots are being explored. These sustained-release systems represent the next technological frontier, promising reduced side effect profiles by maintaining steady drug concentration and eliminating the peak-and-trough concentration variability associated with traditional drops.
Digital health and telemedicine platforms also form an increasingly important part of the Durezol market technology landscape, albeit indirectly. These technologies enable remote monitoring of patients post-surgery, allowing practitioners to track symptoms, manage complications like elevated IOP, and assess inflammatory status without an in-person visit. Such remote capabilities enhance the safety profile of potent steroids like Durezol by facilitating timely intervention if side effects occur, thereby expanding the confidence of ophthalmologists in prescribing the drug outside of immediate hospital settings. Furthermore, integrating these digital tools with pharmacy systems ensures prescription accuracy and adherence tracking, optimizing overall post-operative care management and supporting the rational use of the product.
Regional Highlights
- North America (USA and Canada)
North America holds the largest market share in the Durezol market, primarily driven by high disposable incomes, advanced healthcare infrastructure, and established reimbursement mechanisms. The United States, in particular, exhibits high surgical volumes, especially cataract procedures, necessitating effective post-operative management. The market is characterized by intense competition between branded Durezol and its generic equivalents (generic difluprednate). High prescription volumes are channeled through large ophthalmology practices and Ambulatory Surgical Centers (ASCs). Market success in this region depends heavily on maintaining strong formulary status with major insurance providers and demonstrating clinical superiority via robust comparative effectiveness studies against alternative anti-inflammatory agents. Pricing is premium, though constantly challenged by PBM negotiations, requiring continuous investment in promotional efforts that highlight the clinical benefits of the emulsion formulation.
- Europe (Germany, UK, France, Italy, Spain)
The European market for Durezol is stable but heavily influenced by national healthcare systems and centralized procurement policies. Countries like the UK and Germany prioritize cost-effectiveness, often leading to rapid uptake of generic alternatives once available. However, in specialty prescribing (e.g., complex uveitis cases), the branded product maintains strength due to physician familiarity and perceived quality. The presence of major pharmaceutical manufacturing hubs in countries like Ireland and Switzerland supports the overall ophthalmic drug supply chain. Growth acceleration in Europe requires navigating varied regulatory pathways (EMA approval followed by national price negotiations) and proving long-term cost savings through reduced complication rates, a metric highly valued by public health bodies.
- Asia Pacific (APAC) (China, Japan, India, South Korea)
APAC is projected to be the fastest-growing region during the forecast period. This growth is fueled by massive, underserved populations, rapidly improving healthcare access, and the rising prevalence of age-related eye conditions due to demographic shifts. Countries like China and India are seeing substantial increases in annual cataract surgeries. While price sensitivity remains high, there is a growing segment of patients and practitioners willing to adopt premium, effective drugs like Durezol for superior outcomes. Market expansion in APAC necessitates localized manufacturing, tailored pricing strategies to fit varied income levels, and significant investment in professional education to familiarize local ophthalmologists with the benefits of advanced emulsion steroids over traditional suspension formulations.
- Latin America (LATAM) (Brazil, Mexico, Argentina)
The LATAM market offers moderate growth potential, constrained by economic volatility and complex regulatory systems, but balanced by large public health needs and a growing private healthcare sector. Market penetration often relies on government tenders and securing favorable pricing agreements through national drug agencies. Brazil and Mexico represent the largest opportunities due to their relative stability and increasing health expenditure. The primary challenge is combating unauthorized imports and ensuring reliable cold chain logistics for pharmaceutical distribution across vast geographical distances. Education focusing on the clinical utility in post-operative management is key to expanding prescribing habits beyond major metropolitan centers.
- Middle East & Africa (MEA) (GCC Countries, South Africa)
The MEA region is characterized by high-value specialty drug markets in the Gulf Cooperation Council (GCC) nations, driven by high per capita healthcare spending and modern infrastructure, resulting in high uptake of branded pharmaceuticals. Conversely, the African continent presents significant challenges regarding infrastructure, affordability, and accessibility. Durezol uptake is strongest in countries like UAE and Saudi Arabia, where patients often seek out the latest therapeutic options. Market strategy in MEA focuses on specialized distribution networks and direct engagement with key opinion leaders (KOLs) and private hospital groups.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Durezol Market.- Bausch + Lomb (Original Brand Holder/Developer)
- Akorn Operating Company (Manufacturer of Generic Difluprednate)
- Sandoz (Novartis Group)
- Teva Pharmaceutical Industries Ltd.
- Sun Pharmaceutical Industries Ltd.
- Alcon Inc.
- Santen Pharmaceutical Co., Ltd.
- Allergan (an AbbVie Company)
- Pfizer Inc.
- Glaukos Corporation
- Ocular Therapeutix, Inc. (Focusing on sustained-release technology)
- Amgen Inc.
- Bristol-Myers Squibb Company
- Johnson & Johnson (Janssen)
- Cipla Ltd.
- Mylan N.V. (Now Viatris)
- Bayer AG
- F. Hoffmann-La Roche AG
- Regeneron Pharmaceuticals, Inc.
Frequently Asked Questions
Analyze common user questions about the Durezol market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is Durezol primarily used for in the ophthalmic market?
Durezol (difluprednate ophthalmic emulsion) is primarily used for treating inflammation and pain that occur following ocular surgery, most commonly cataract extraction. It is also indicated for the management of endogenous anterior uveitis.
How does the generic entry of difluprednate impact the Durezol market share?
Generic entry creates significant pricing pressure, leading to market share erosion for the branded product, particularly in formulary-driven environments. However, Durezol maintains value by emphasizing its established brand loyalty, unique formulation characteristics, and extensive clinical history.
What is the key advantage of Durezol's emulsion formulation over older steroid suspensions?
The key advantage of Durezol’s emulsion formulation is enhanced ocular penetration and bioavailability, allowing for less frequent dosing (typically twice daily) compared to older steroid suspensions, which improves patient adherence and therapeutic outcomes.
Which geographical region exhibits the highest growth potential for the Durezol market?
The Asia Pacific (APAC) region, including major economies like China and India, is expected to exhibit the highest growth potential due to increasing surgical volumes, improving healthcare infrastructure, and rising adoption of advanced post-operative care protocols.
Are sustained-release technologies expected to replace traditional Durezol drops?
Sustained-release technologies, such as drug-eluting punctal plugs containing difluprednate, are being developed and commercialized. While they offer superior adherence and convenience, they are more likely to serve as premium alternatives rather than completely replacing traditional Durezol drops, initially targeting high-risk or non-compliant patients.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager