Febuxostat Market Size, By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 442271 | Date : Feb, 2026 | Pages : 241 | Region : Global | Publisher : MRU
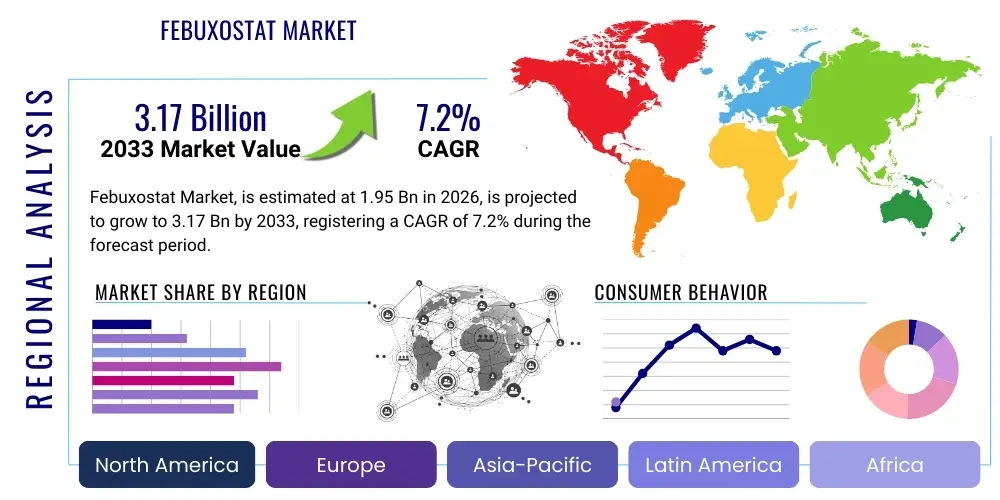
Febuxostat Market Size
The Febuxostat Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.2% between 2026 and 2033. The market is estimated at $1.95 Billion in 2026 and is projected to reach $3.17 Billion by the end of the forecast period in 2033.
Febuxostat Market introduction
Febuxostat, a non-purine selective inhibitor of xanthine oxidase (XOI), represents a critical therapeutic agent primarily used for the management of chronic hyperuricemia in adult patients with gout. Gout, a prevalent inflammatory arthritic condition resulting from the deposition of urate crystals, requires effective long-term management to prevent acute flares and associated joint damage. Febuxostat offers an alternative to traditional treatments like allopurinol, particularly benefiting patients who exhibit intolerance or inadequate response to the established purine-based inhibitors, thereby expanding the treatment landscape for this chronic condition globally.
The product’s efficacy stems from its mechanism of action: lowering serum uric acid (sUA) levels by inhibiting the enzyme responsible for uric acid production. Major applications of Febuxostat revolve around prophylactic treatment to reduce the incidence of gout flares and the dissolution of urate crystals. The primary benefits include its non-purine structure, which often results in fewer drug interactions compared to older XOI therapies, and its high effectiveness in achieving target sUA levels. Furthermore, Febuxostat offers dose flexibility and does not typically require dose adjustments based on renal function, unlike some alternatives, making it highly advantageous for patients with mild-to-moderate renal impairment.
Driving factors propelling the Febuxostat market expansion include the rising global prevalence of gout, fueled by aging populations and increasing rates of lifestyle diseases such as obesity, hypertension, and chronic kidney disease, which are significant risk factors for hyperuricemia. Coupled with growing awareness among patients and healthcare providers regarding the importance of sustained sUA control to prevent long-term complications, the demand for effective and well-tolerated treatments like Febuxostat continues to escalate. Market growth is further sustained by ongoing research into its potential role in managing hyperuricemia-related cardiovascular risks and the strategic expansion of generic formulations in developing economies.
Febuxostat Market Executive Summary
The Febuxostat market demonstrates robust growth driven by high unmet needs in chronic gout management and favorable clinical efficacy profiles compared to older therapies. Business trends indicate a shift towards generic dominance following patent expiry in major economies, which, while pressuring average selling prices, significantly expands market access and patient uptake, particularly in price-sensitive regions. Strategic collaborations focused on post-marketing surveillance and expanding indication coverage, alongside intense competition among pharmaceutical manufacturers, define the current business landscape. Key manufacturers are investing heavily in improving drug delivery mechanisms and combination therapies to maintain competitive advantage.
Regional trends reveal North America and Europe as established revenue centers, characterized by high treatment expenditure and sophisticated reimbursement frameworks. However, the fastest growth trajectory is anticipated in the Asia Pacific (APAC) region, stemming from rapidly increasing geriatric populations, urbanization leading to higher prevalence of lifestyle diseases linked to gout, and improving healthcare infrastructure facilitating better diagnosis and prescription rates. Middle East and Africa (MEA) present nascent but significant opportunities, particularly as economic growth enables broader access to branded and high-quality generic Febuxostat formulations.
Segment trends underscore the dominance of the 80 mg dosage segment due to its common use in maintenance therapy following initial dose escalation. Furthermore, the segmentation by distribution channel highlights the critical role of hospital pharmacies in initial prescription fulfillment, transitioning to retail and online pharmacies for long-term patient refill needs. The increasing focus on patient adherence and convenience is also stimulating innovation in fixed-dose combination products aimed at simplifying gout management regimens, thereby bolstering segment diversification and market resilience against single-product reliance.
AI Impact Analysis on Febuxostat Market
User inquiries concerning AI's influence on the Febuxostat market predominantly revolve around its potential for accelerating personalized medicine, optimizing clinical trial design for new formulations or combination therapies, and enhancing diagnostic accuracy for hyperuricemia and associated comorbidities. Key themes highlight expectations for AI-driven identification of high-risk patient subgroups who may benefit most from Febuxostat versus allopurinol, and concerns about data privacy when using predictive analytics based on electronic health records (EHRs) for treatment stratification. Users also anticipate AI being utilized for drug discovery processes, potentially identifying novel XOI targets or predicting patient responses to Febuxostat based on genetic markers, thereby improving treatment outcomes and reducing adverse drug reactions.
- AI accelerates patient stratification for Febuxostat therapy, distinguishing responders from non-responders using machine learning algorithms applied to clinical and genomic data.
- Predictive modeling enhances precision in monitoring serum uric acid levels, allowing for proactive dose adjustments and improved patient adherence.
- AI-driven analysis of real-world evidence (RWE) identifies previously unrecognized cardiovascular or renal benefits associated with Febuxostat use.
- Automation in pharmacovigilance detects rare adverse events linked to Febuxostat faster than traditional surveillance methods.
- Optimization of clinical trial recruitment reduces the time and cost associated with developing new Febuxostat delivery systems or combination products.
DRO & Impact Forces Of Febuxostat Market
The Febuxostat market is significantly shaped by compelling drivers, including the increasing global burden of gout and associated hyperuricemia, coupled with clinical guidelines recommending stringent management of serum uric acid levels to prevent debilitating joint damage. The product's superior efficacy and tolerability profile in certain patient populations, especially those with allopurinol hypersensitivity or suboptimal response, serve as strong market accelerators. Furthermore, strategic shifts toward preventive care and chronic disease management globally reinforce the long-term demand for effective urate-lowering therapies (ULTs). These drivers collectively create a buoyant market environment.
However, the market faces notable restraints, primarily the intense generic competition following the expiry of key patents in major markets, which compresses profitability margins for branded manufacturers. Regulatory scrutiny and boxed warnings related to cardiovascular safety concerns, although often debated in clinical circles, continue to influence physician prescribing behavior, particularly in the United States. High treatment costs in certain regions, despite the availability of generics, can also limit patient access and adherence, especially in healthcare systems with limited public funding for chronic disease management. These restraints necessitate innovative pricing and market access strategies.
Opportunities abound, particularly in emerging markets where diagnosis rates for gout are rapidly increasing and healthcare expenditure is rising. Developing fixed-dose combinations of Febuxostat with anti-inflammatory agents for acute flare management, or exploring its application in managing hyperuricemia secondary to chemotherapy (tumor lysis syndrome), present lucrative avenues for market expansion. The impact forces—ranging from robust clinical evidence supporting long-term efficacy to favorable demographic trends characterized by increased risk factors for gout—ensure sustained demand, while regulatory changes and competitor actions require continuous strategic adaptation.
Segmentation Analysis
The Febuxostat market is systematically segmented based on dosage strength, indication, and distribution channel, providing a granular view of market dynamics and commercial viability across various end-user requirements. Dosage strength segmentation is crucial, reflecting standard clinical protocols where patients often start with a lower dose (40 mg) and titrate up to a higher maintenance dose (80 mg or 120 mg) to achieve target serum uric acid levels. This segmentation is vital for manufacturers planning production volumes and for distributors managing inventory, directly impacting patient adherence and therapeutic outcomes.
Segmentation by indication primarily focuses on chronic management of hyperuricemia in adult patients with gout, the main approved use. However, emerging off-label uses or research into secondary hyperuricemia related to other conditions offers future expansion potential. Distribution channels—encompassing hospital pharmacies, retail pharmacies, and online sales—reflect the patient journey, from initial diagnosis and prescription (often in a hospital setting) to long-term chronic management (via retail or online platforms). The growing penetration of e-commerce platforms, particularly in developed markets, is influencing the distribution landscape toward greater convenience and accessibility.
Understanding these segments allows market participants to tailor their marketing strategies, focusing resources on the most profitable dosage strengths or the fastest-growing distribution channels. The 80 mg segment is typically the largest volume generator globally due to its established role as the standard maintenance dose. The increasing acceptance of high-quality generic versions is redefining competition within these segments, shifting the focus from proprietary formulations to cost-effectiveness and supply chain reliability. This structured approach to segmentation is crucial for accurate forecasting and strategic planning.
- By Dosage Strength:
- 40 mg
- 80 mg
- 120 mg
- By Indication:
- Chronic Management of Hyperuricemia in Gout Patients
- Others (e.g., potential applications in tumor lysis syndrome, research settings)
- By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- By Region:
- North America
- Europe
- Asia Pacific (APAC)
- Latin America (LATAM)
- Middle East and Africa (MEA)
Value Chain Analysis For Febuxostat Market
The value chain for the Febuxostat market begins with upstream activities focused on the sourcing and synthesis of active pharmaceutical ingredients (APIs). API manufacturing requires stringent quality control and specialized chemical processes, often involving complex organic synthesis steps. Key challenges upstream include ensuring the stability and purity of the chemical compounds, managing fluctuations in raw material prices, and navigating complex intellectual property landscapes, especially for patented synthesis routes. Companies strive for vertical integration or strong long-term contracts with specialized Contract Manufacturing Organizations (CMOs) to secure reliable and cost-effective API supply, which is critical given the high demand for both branded and generic finished products.
Midstream activities involve the formulation of the drug into finished dosage forms (tablets), quality assurance testing, and packaging. This stage is dominated by large pharmaceutical companies and generic manufacturers who operate sophisticated manufacturing facilities compliant with Good Manufacturing Practices (GMP). Efficient formulation development is essential to enhance bioavailability and stability. The downstream segment focuses on distribution, marketing, and sales. Distribution channels are highly regulated, involving both direct sales to major hospital systems and indirect sales through wholesale distributors who supply retail and hospital pharmacies. Wholesalers play a critical role in inventory management and ensuring timely delivery across vast geographical areas.
Direct distribution often targets major institutional buyers and government tenders, allowing manufacturers greater control over pricing and access. Indirect distribution, leveraging wholesalers and pharmacy benefit managers (PBMs), facilitates broader market penetration to individual retail consumers. The rise of specialized pharmacy services and mail-order pharmacies, particularly in chronic disease management like gout, represents an increasingly important facet of downstream optimization. The entire value chain is anchored by research and development (R&D) and clinical trials, which validate efficacy and safety, maintaining the product’s commercial viability and supporting its market positioning against competitors.
Febuxostat Market Potential Customers
The primary end-users and buyers of Febuxostat are adult patients diagnosed with chronic hyperuricemia and symptomatic gout, particularly those who are intolerant to or inadequately controlled by traditional first-line treatments like allopurinol. This patient demographic often includes individuals over the age of 40, predominantly male, with high rates of associated metabolic syndromes such as diabetes, hypertension, and renal impairment. Due to the chronic nature of the disease, these customers require sustained, long-term medication, making adherence programs and convenient dosing regimens crucial considerations for market stakeholders. The need for Febuxostat is further pronounced in patient groups where lowering serum uric acid levels below the typical threshold (e.g., 5 mg/dL) is necessary to dissolve tophi.
Secondary but significant customers include geriatric populations, as the prevalence of gout increases substantially with age. Furthermore, institutional purchasers, such as hospitals, government healthcare systems, and health maintenance organizations (HMOs), represent major buying entities. These large organizations procure Febuxostat in bulk, often via tenders or formulary contracts, based on cost-effectiveness, clinical guidelines, and population health management goals. Specialty clinics focused on rheumatology and internal medicine are also critical points of prescription and consumption, serving as key opinion leaders (KOLs) who influence treatment patterns.
In the context of the pharmaceutical supply chain, the immediate purchasers include retail pharmacies, hospital pharmacies, and wholesale distributors. These entities act as conduits, facilitating the availability of Febuxostat to the final consumers. The decisions of PBMs and insurance providers are also critical, as their formulary placement and coverage policies directly determine patient out-of-pocket costs and overall market uptake. Consequently, marketing efforts must target not only prescribers (rheumatologists, internists, and primary care physicians) but also organizations that control access and reimbursement for this essential chronic care medication.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | $1.95 Billion |
| Market Forecast in 2033 | $3.17 Billion |
| Growth Rate | 7.2% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Takeda Pharmaceutical Company Limited, Astellas Pharma Inc., TEVA Pharmaceutical Industries Ltd., Mylan N.V. (now Viatris), Sun Pharmaceutical Industries Ltd., Zydus Lifesciences Limited, Hetero Drugs, Cipla Ltd., Dr. Reddy's Laboratories, Aurobindo Pharma, Accord Healthcare, Intas Pharmaceuticals Ltd., Hikma Pharmaceuticals PLC, Sandoz (Novartis AG), Amneal Pharmaceuticals Inc., Lannett Company Inc., Alkem Laboratories, Lupin Limited, Bristol-Myers Squibb Company. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Febuxostat Market Key Technology Landscape
The technology landscape surrounding the Febuxostat market primarily focuses on enhancing drug delivery, improving patient adherence, and optimizing manufacturing processes for cost efficiency. While Febuxostat itself is a conventional oral small molecule, technological advancements are being channeled into creating modified-release formulations that might improve patient compliance by reducing dosing frequency or offering targeted release in the gastrointestinal tract. Furthermore, solid oral dosage manufacturing technologies, such as advanced granulation techniques and continuous manufacturing processes, are being deployed by generic players to ensure consistent product quality, high volume throughput, and rapid scale-up capabilities, which are essential for competing effectively in this high-volume generic market.
Beyond formulation and manufacturing, digital health technologies are playing an increasing role in patient management. This includes the development of mobile health (mHealth) applications designed to track patient adherence, monitor symptom severity, and remind patients about necessary laboratory tests for monitoring serum uric acid levels. These integrated digital solutions leverage data analytics to provide personalized feedback and improve patient-physician communication regarding chronic gout management. These technological aids are vital in overcoming the challenge of poor long-term adherence often observed in chronic disease therapy, thereby maximizing the therapeutic benefit of Febuxostat.
Furthermore, analytical technologies are crucial for quality control and bioequivalence testing, particularly in the highly competitive generic sector. High-performance liquid chromatography (HPLC), mass spectrometry (MS), and dissolution testing equipment are standard tools used throughout the production life cycle to ensure that generic Febuxostat formulations meet the strict regulatory requirements for purity, potency, and absorption profiles relative to the reference listed drug. Innovation in these analytical methods allows for faster batch release and robust quality documentation, supporting the global expansion of Febuxostat generics into regulated markets.
Regional Highlights
- North America: North America, particularly the United States, holds a significant share of the Febuxostat market revenue, driven by high per capita healthcare spending, established reimbursement systems, and high awareness regarding the necessity of treating hyperuricemia. Despite early patent expirations and the presence of low-cost generics, the region commands high value due to complex distribution channels and the strong influence of key opinion leaders and clinical guidelines advocating for individualized treatment based on patient response to allopurinol. The market here is characterized by intense price negotiation between manufacturers and PBMs, focusing heavily on formulary placement and market access strategies.
- Europe: Western European countries represent a mature market segment, characterized by centralized healthcare systems and rigorous regulatory environments. Market growth is sustained by an aging population and high prevalence of risk factors for gout. Febuxostat is widely adopted, often listed favorably in national treatment guidelines, especially for patients with renal impairment. Pricing pressure is substantial, primarily driven by tendering processes and the widespread availability of generics, necessitating manufacturers to focus on lifecycle management and differentiation through service offerings or patient support programs.
- Asia Pacific (APAC): APAC is projected to be the fastest-growing region, fueled by massive population bases, increasing disposable income, and rapid improvements in healthcare infrastructure in countries like China and India. The shift toward Westernized diets and lifestyle changes is rapidly escalating the incidence of gout and hyperuricemia. Market entry strategies often focus on establishing partnerships and navigating diverse regulatory landscapes. While generics dominate the volume, branded segments see opportunities in catering to premium, self-paying patient demographics demanding imported or higher-perceived quality medications.
- Latin America (LATAM): LATAM markets are undergoing modernization, with expanding public health coverage improving access to chronic disease treatments. Febuxostat adoption is rising, though market growth is often constrained by economic volatility and complex drug registration processes. Brazil and Mexico are key growth engines, driven by private sector growth and increasing diagnostic rates. Manufacturers often employ localized pricing strategies to ensure affordability and increase penetration against competitive generic offerings.
- Middle East and Africa (MEA): The MEA region offers emerging opportunities, especially in the Gulf Cooperation Council (GCC) states where high-quality healthcare investment is significant. Lifestyle-related diseases, including gout, are on the rise. Market penetration is steadily increasing, focusing primarily on high-income patient groups and specialized hospital systems. Challenges include fragmented distribution channels and the need for localized clinical education to promote optimal Febuxostat use according to international standards.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Febuxostat Market.- Takeda Pharmaceutical Company Limited
- Astellas Pharma Inc.
- TEVA Pharmaceutical Industries Ltd.
- Mylan N.V. (now Viatris)
- Sun Pharmaceutical Industries Ltd.
- Zydus Lifesciences Limited
- Hetero Drugs
- Cipla Ltd.
- Dr. Reddy's Laboratories
- Aurobindo Pharma
- Accord Healthcare
- Intas Pharmaceuticals Ltd.
- Hikma Pharmaceuticals PLC
- Sandoz (Novartis AG)
- Amneal Pharmaceuticals Inc.
- Lannett Company Inc.
- Alkem Laboratories
- Lupin Limited
Frequently Asked Questions
Analyze common user questions about the Febuxostat market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary function and market position of Febuxostat in gout treatment?
Febuxostat is a highly selective non-purine xanthine oxidase inhibitor (XOI) used to manage chronic hyperuricemia in adults with gout. It holds a crucial market position as an effective second-line therapy or preferred option for patients intolerant to or unresponsive to allopurinol, offering superior efficacy in achieving target serum uric acid levels.
How is the global Febuxostat market impacted by generic competition?
Generic competition following patent expiry has significantly lowered the average selling price (ASP) of Febuxostat globally. This price erosion expands patient access, especially in emerging markets, but places intense margin pressure on branded and generic manufacturers, driving them towards manufacturing efficiencies and market consolidation.
What are the key safety considerations affecting Febuxostat market adoption?
The key safety consideration is the potential cardiovascular risk, leading to regulatory scrutiny and boxed warnings in some jurisdictions (e.g., the FDA). While clinical debate continues, these warnings necessitate careful patient selection and monitoring, influencing physician prescribing habits and restricting adoption in certain high-risk cardiovascular patient demographics.
Which geographical region is expected to demonstrate the fastest growth rate for Febuxostat?
The Asia Pacific (APAC) region is projected to exhibit the fastest market growth. This acceleration is attributed to the rapidly increasing prevalence of gout linked to changing lifestyles, coupled with expanding geriatric populations and significant ongoing investment in healthcare infrastructure and access across major economies like China and India.
What are the primary segments driving market revenue for Febuxostat?
The market revenue is primarily driven by the 80 mg dosage strength segment, which is the widely accepted standard for long-term maintenance therapy in chronic gout patients. Revenue generation is heavily reliant on retail and hospital pharmacies, serving the massive patient pool requiring continuous uric acid level management.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager