Hepatitis A Vaccine Market Size, By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 443570 | Date : Feb, 2026 | Pages : 245 | Region : Global | Publisher : MRU
Hepatitis A Vaccine Market Size
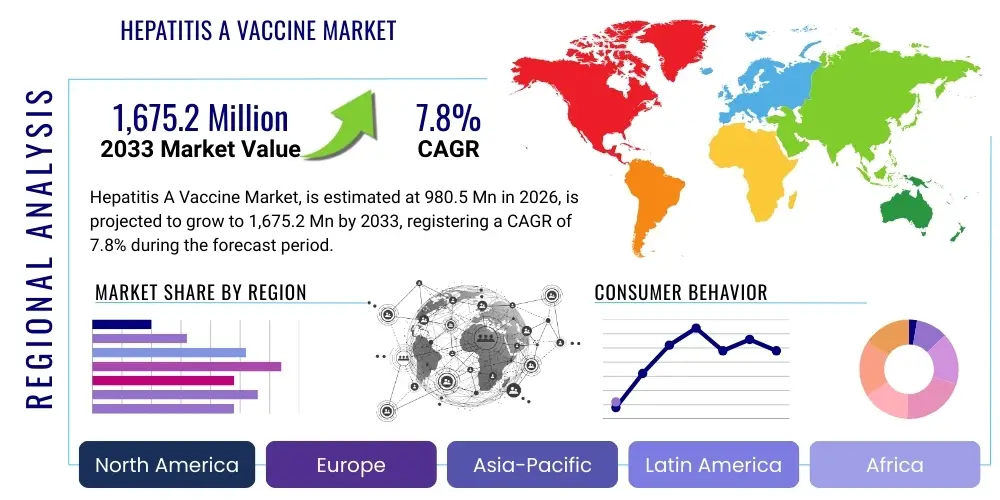
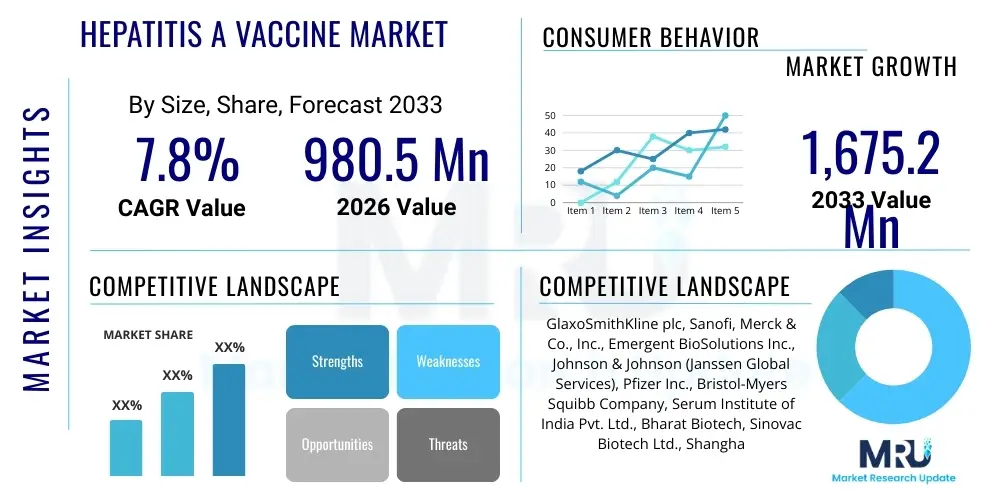
The Hepatitis A Vaccine Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.8% between 2026 and 2033. The market is estimated at USD 980.5 Million in 2026 and is projected to reach USD 1,675.2 Million by the end of the forecast period in 2033.
Hepatitis A Vaccine Market introduction
The global Hepatitis A Vaccine market encompasses the development, manufacturing, and distribution of pharmaceutical agents designed to prevent infection caused by the Hepatitis A virus (HAV). HAV is an RNA virus transmitted primarily through the fecal-oral route, typically via contaminated food or water, leading to acute liver inflammation. The vaccines available are highly effective, providing long-term immunity against this preventable disease. Market expansion is fundamentally driven by increasing governmental and non-governmental organization (NGO) initiatives aimed at broadening immunization programs, particularly in regions with high endemicity where sanitation standards remain challenging.
The primary products dominating this market are monovalent Hepatitis A vaccines and combination vaccines, such as those combining Hepatitis A and Hepatitis B antigens. These vaccines are essential components of global public health strategies, targeting both vulnerable populations in developed nations and large-scale pediatric immunization programs in developing countries. Key applications involve routine childhood immunization schedules, vaccination for travelers visiting high-risk areas, and protective measures for specific occupational risk groups like sanitation workers and healthcare professionals. The efficacy and safety profile of existing vaccines have solidified their acceptance globally, prompting consistent demand.
Major benefits derived from widespread Hepatitis A vaccination include the substantial reduction in incidence rates, the prevention of costly hospitalizations and long-term liver complications, and the mitigation of large-scale epidemic outbreaks, especially those linked to food contamination events. The driving factors propelling market growth include mandatory vaccination policies in several developed nations, rising awareness concerning foodborne and waterborne diseases, continuous innovation in manufacturing processes to improve vaccine thermostability and accessibility, and aggressive procurement strategies by international health bodies such as WHO and UNICEF for mass vaccination campaigns in underserved areas.
Hepatitis A Vaccine Market Executive Summary
The Hepatitis A Vaccine market demonstrates robust growth trajectory, underpinned by escalating public health investments and mandatory pediatric immunization mandates across major economies. Current business trends indicate a strong shift toward combined prophylactic approaches, favoring vaccines that offer protection against multiple strains or diseases, thereby improving patient compliance and simplifying administration logistics. Key market players are concentrating on expanding manufacturing capacities in emerging economies and focusing on research to develop needle-free or improved stability formulations to enhance global distribution efficiency. Strategic collaborations between pharmaceutical giants and regional distribution partners are critical for penetrating highly regulated or epidemiologically sensitive markets, solidifying a competitive landscape characterized by quality and supply chain resilience.
Regional trends significantly influence market dynamics, with North America and Europe maintaining dominant market shares due to high healthcare expenditure, established immunization protocols, and robust public awareness campaigns targeting vulnerable groups such as men who have sex with men (MSM) and individuals with chronic liver disease. Concurrently, the Asia Pacific (APAC) region is poised for the fastest expansion, driven by immense population density, improving sanitation infrastructure that paradoxically increases susceptibility in older populations, and governmental commitment to nationwide pediatric vaccination programs. Latin America and the Middle East and Africa (MEA) present substantial untapped potential, contingent upon addressing challenges related to cold chain maintenance and affordability, often relying heavily on global aid initiatives for vaccine supply.
Segmentation analysis reveals that the inactivated vaccine segment, utilizing traditional manufacturing methods, remains the preferred product type globally due to its established safety record and widespread regulatory acceptance. However, advancements in combination vaccines are gaining traction, appealing to providers seeking streamlined immunization schedules. By dosage, the pediatric segment consistently commands a larger volume share owing to routine childhood immunization mandates, while the adult segment, fueled by travel health and high-risk group vaccination recommendations, generates significant revenue due to higher unit pricing and specific demographic targeting. Distribution channels are diversifying, with government procurement agencies playing a pivotal role in mass purchasing, complemented by steady growth through private hospital and retail pharmacy channels, ensuring accessibility across different socio-economic strata.
AI Impact Analysis on Hepatitis A Vaccine Market
Analysis of common user questions regarding AI's impact on the Hepatitis A Vaccine market reveals key themes centered around four main areas: the use of predictive analytics for outbreak forecasting, optimization of vaccine development timelines, enhancement of manufacturing efficiency, and improvement of supply chain logistics, particularly cold chain management. Users frequently inquire about how AI can accelerate the identification of optimal antigen candidates or adjuvants to improve immunogenicity. A significant concern is also focused on leveraging machine learning models to analyze complex epidemiological data patterns, enabling public health bodies to deploy targeted vaccination campaigns proactively before large-scale outbreaks occur, thereby maximizing resource allocation efficiency and reducing the severity of epidemics.
The summary of these analyses indicates a clear expectation that AI will revolutionize the market primarily through predictive epidemiology and manufacturing optimization. AI algorithms can process vast amounts of environmental data, travel patterns, and sanitation metrics to accurately predict HAV hotspots, allowing for preemptive vaccine deployment, moving from reactive containment to proactive prevention. Furthermore, in the highly regulated manufacturing sector, AI is expected to streamline quality control, optimize fermentation and purification processes, and model complex protein folding, potentially shortening the laborious and time-intensive production cycles associated with vaccine manufacturing, thereby improving global vaccine availability and reducing manufacturing costs, which is crucial for affordability in emerging markets.
- AI-driven predictive epidemiology enables precise forecasting of Hepatitis A outbreaks and localized risk areas.
- Machine learning models accelerate R&D by identifying novel epitopes and optimizing vaccine design for enhanced efficacy.
- Optimization of vaccine manufacturing processes, including yield maximization and reduced quality control turnaround times.
- Enhanced cold chain logistics management using AI to monitor temperature fluctuations and predict supply chain vulnerabilities.
- Data analysis of post-marketing surveillance for real-time detection of adverse events and continuous safety monitoring.
DRO & Impact Forces Of Hepatitis A Vaccine Market
The Hepatitis A Vaccine market dynamics are shaped by a complex interplay of growth drivers, inherent constraints, and significant opportunities, which collectively define the impact forces influencing market trajectory. The primary driver is the widespread implementation of mandatory childhood immunization programs and the sustained risk of sporadic and large-scale outbreaks, especially in populous regions with fluctuating sanitation standards. However, market expansion is restrained by the high initial cost of vaccines in certain economies, coupled with significant challenges in maintaining the stringent cold chain requirements essential for vaccine stability, particularly during distribution across vast and remote geographical areas, leading to potential wastage and reduced efficacy in peripheral markets.
Opportunities for market growth are abundant, notably through the penetration of previously untapped rural and low-income markets facilitated by government and global health organization procurement tenders. Furthermore, continuous development in combination vaccines, integrating Hepatitis A protection with other vital childhood immunizations (e.g., Hepatitis B, Typhoid), offers a substantial commercial pathway by improving compliance and reducing the burden on healthcare systems. These opportunities are highly attractive to pharmaceutical companies seeking portfolio diversification and seeking to establish long-term supply agreements with national health ministries, ensuring sustained revenue streams and enhanced public health impact.
The impact forces within this ecosystem are substantial: regulatory stringency dictates R&D investment and manufacturing practices, high switching costs for established immunization protocols limit rapid adoption of new formulations, and competitive pressure among the few large manufacturers influences pricing and supply capacity. The overall momentum suggests that while regulatory hurdles and supply chain fragility remain constraints, the overwhelming public health mandate and increasing global travel rates act as powerful, sustained drivers, forcing manufacturers to innovate in formulation stability and expand their geographical reach to meet the burgeoning global demand for effective HAV prophylaxis.
Segmentation Analysis
The Hepatitis A Vaccine market is segmented based on critical attributes including product type, dosage regimen, route of administration, and distribution channel, providing a granular view of market dynamics and revenue streams. Segmentation by product type highlights the historical dominance of inactivated vaccines due to their robust safety profile and established manufacturing processes, although newer live attenuated variants are under development for specific regional applications. Dosage regimen clearly differentiates between the higher volume pediatric segment, driven by routine immunization schedules, and the high-value adult segment, which caters to travelers, high-risk occupational groups, and individuals with underlying liver conditions, reflecting distinct pricing strategies and market access mechanisms.
- By Product Type:
- Inactivated Vaccines (Formalin-inactivated)
- Live Attenuated Vaccines (Limited regional use and R&D focus)
- Combination Vaccines (e.g., Hep A & Hep B)
- By Dosage:
- Pediatric Dosage
- Adult Dosage
- By Route of Administration:
- Intramuscular Injection (Standard)
- By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Government & Public Health Procurement Agencies
- Others (NGOs, Clinics)
Value Chain Analysis For Hepatitis A Vaccine Market
The value chain for the Hepatitis A Vaccine market begins with intensive upstream activities focused on antigen preparation and purification, encompassing highly specialized biological processes involving cell culture and inactivation methods, where compliance with Good Manufacturing Practices (GMP) is paramount. This initial stage requires significant capital investment in bioreactors, sophisticated analytical instrumentation, and highly skilled personnel. Major pharmaceutical companies often vertically integrate or maintain strict control over this upstream segment to ensure quality, consistency, and stability of the vaccine bulk. The raw material suppliers, including media manufacturers and adjuvant providers, must meet exacting standards, forming a crucial foundation for the subsequent manufacturing stages.
The midstream phase involves filling, finishing, and packaging, transforming the bulk vaccine into final marketable doses suitable for cold chain distribution. This process includes vialing, labeling, and robust secondary packaging to protect temperature sensitivity, demanding high-speed, automated equipment under sterile conditions. Downstream activities are dominated by complex logistics and distribution channels, involving both direct sales to government procurement bodies (often through competitive tendering processes) and indirect distribution through wholesalers, specialized cold chain logistics providers, and hospital pharmacies. The government and public health channels represent the largest volume segment, driving the necessity for efficient, high-volume manufacturing capabilities among market leaders.
Distribution channels for Hepatitis A vaccines are bifurcated into direct and indirect routes. Direct distribution involves large-scale agreements with national immunization programs (NIPs) and international organizations like WHO, where volumes are high and pricing is often negotiated based on public health objectives. Indirect channels involve commercial sales to private healthcare providers, retail pharmacies, and travel clinics, offering higher margins but smaller individual volumes. The integrity of the cold chain, maintaining temperatures typically between 2°C and 8°C throughout the complex distribution network, is the single most critical factor in the downstream segment, heavily influencing market accessibility and product viability in diverse climatic zones.
Hepatitis A Vaccine Market Potential Customers
The potential customer base for Hepatitis A vaccines is extensive, segmented primarily into public health programs, private healthcare consumers, and institutional purchasers. The largest buyer segment is represented by governmental health agencies and international non-profit organizations (e.g., UNICEF, PAHO), which purchase vaccines in massive volumes for routine pediatric immunization schedules and outbreak control strategies. These entities are characterized by price sensitivity and demanding requirements regarding consistent supply, extended shelf life, and WHO prequalification status, making them the backbone of global vaccine demand, particularly in Asia Pacific and Africa where endemicity is high.
A second major customer group includes individual private consumers accessing vaccines through private clinics, retail pharmacies, and travel health centers. This segment encompasses international travelers, especially those visiting endemic regions, individuals with pre-existing liver conditions (e.g., chronic Hepatitis B or C), and populations considered high-risk due to behavioral factors or occupational exposure (e.g., laboratory personnel, food handlers). These customers prioritize convenience, brand reputation, and availability of combined vaccines, and are less price-sensitive than government procurers, contributing significantly to the adult dosage revenue stream in developed markets like North America and Western Europe.
Finally, institutional customers, such as large hospital systems, occupational health providers, military organizations, and major food service corporations, constitute a specialized purchasing segment. Hospitals procure for patient treatment and staff protection, while corporate entities often mandate vaccination for employees in high-risk roles or those involved in international deployment. These customers prioritize logistical support, ease of integration into existing healthcare protocols, and reliability of supply, often engaging in direct contractual arrangements with manufacturers or large distributors to ensure timely access to necessary prophylactic measures.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 980.5 Million |
| Market Forecast in 2033 | USD 1,675.2 Million |
| Growth Rate | 7.8% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | GlaxoSmithKline plc, Sanofi, Merck & Co., Inc., Emergent BioSolutions Inc., Johnson & Johnson (Janssen Global Services), Pfizer Inc., Bristol-Myers Squibb Company, Serum Institute of India Pvt. Ltd., Bharat Biotech, Sinovac Biotech Ltd., Shanghai Institute of Biological Products, Takeda Pharmaceutical Company Limited, Dynavax Technologies Corporation, Mitsubishi Tanabe Pharma Corporation, CSL Limited, Bio Farma (Persero), SK Bioscience Co., Ltd., Crucell N.V. (Part of Johnson & Johnson), Berna Biotech (Part of Crucell/J&J), and Valneva SE. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Hepatitis A Vaccine Market Key Technology Landscape
The core technology driving the Hepatitis A Vaccine market remains the production of inactivated whole-virus vaccines, which relies on well-established cell culture techniques, typically involving the growth of the Hepatitis A virus in human diploid cells or similar substrates, followed by chemical inactivation, often using formalin. This traditional approach ensures high immunogenicity and an excellent safety profile, dominating the commercial landscape globally. Manufacturers are continuously optimizing these bioreactor systems and purification columns to increase yield and reduce batch-to-batch variability, leveraging advancements in bioprocess engineering to scale up production capacity efficiently and meet rising global demand, ensuring consistency in antigen concentration and stability.
Beyond traditional methods, technological innovation is increasingly focusing on enhancing vaccine stability and ease of administration. This includes research into thermostable formulations, often utilizing specialized lyophilization techniques or novel adjuvant systems, aimed at reducing dependency on the complex and costly cold chain, particularly in tropical and resource-limited settings. Successfully developing vaccines that maintain potency at ambient temperatures for extended periods would represent a paradigm shift in global deployment strategies, dramatically improving vaccination coverage in remote areas where reliable refrigeration infrastructure is often lacking or intermittent, thereby expanding market accessibility and public health impact significantly.
Furthermore, the technology landscape is being influenced by the development of sophisticated analytical platforms, including mass spectrometry and advanced chromatography, used for stringent quality control and characterization of the viral antigen structure. Parallel research into next-generation vaccine platforms, such as virus-like particles (VLPs) or recombinant protein-based vaccines, although not yet dominant in the HAV space, holds future potential. These technologies aim to offer improved purity, faster manufacturing timelines, and potentially enhanced cross-protection against minor strain variations, signaling a slow but steady technological transition toward more advanced and scalable production methodologies that could further reduce the cost per dose and enhance global supply resilience.
Regional Highlights
North America: North America holds a substantial share of the global Hepatitis A Vaccine market, characterized by high per capita healthcare spending, universal mandatory childhood immunization programs, and a sophisticated public health surveillance system capable of rapid outbreak response. The market here is driven not only by routine pediatric vaccination but also by significant demand in the adult segment, fueled by specific high-risk populations, including individuals with chronic liver disease, drug users, and the MSM community, as well as high volumes of international travel to endemic regions. Regulatory bodies, primarily the FDA, maintain extremely rigorous standards for vaccine safety and efficacy, leading to market dominance by a few large, established pharmaceutical manufacturers.
The regulatory framework and established infrastructure facilitate premium pricing for both monovalent and combination vaccines, contributing significantly to revenue generation despite comparatively lower endemic rates than other global regions. A key regional trend involves the integration of Hepatitis A vaccination into comprehensive travel medicine protocols and specialized clinics, generating consistent private sector demand. Furthermore, the robust infrastructure ensures minimal cold chain disruption, guaranteeing product quality up to the point of administration. The focus is shifting towards improving vaccination uptake among hard-to-reach adult populations and ensuring compliance with the two-dose schedule necessary for long-term protection, often leveraging advanced electronic health records (EHR) for tracking and recall systems.
The North American market is highly consolidated, with major players aggressively competing through established distribution networks and clinical trial superiority claims. Public awareness campaigns, often run by state health departments and non-profit organizations, play a crucial role in maintaining high vaccination coverage. Investment in surveillance technology, including wastewater epidemiology, is becoming a key area to monitor HAV circulation proactively. This region serves as a benchmark for high-quality vaccine delivery and sustained governmental commitment to eliminating Hepatitis A as a public health threat, demonstrating the financial viability of well-established immunization programs.
Europe: The European market for Hepatitis A vaccines is mature, exhibiting stable growth driven by varied national immunization policies and significant cross-border movement. Western European countries, particularly those in Scandinavia, France, and Germany, have high coverage rates, often incorporating Hepatitis A into optional or targeted immunization schedules, depending on national risk assessments and travel patterns. Eastern European nations, which may experience periodic outbreaks due to varied sanitation standards, rely more heavily on government procurement for catch-up and targeted immunization drives. The presence of the European Medicines Agency (EMA) ensures a harmonized, albeit lengthy, approval process for new vaccine formulations and manufacturing site approvals.
The demand in Europe is substantially influenced by international tourism and migration patterns. With high volumes of citizens traveling to destinations in Africa, Asia, and Latin America, travel health clinics constitute a major consumption channel, leading to strong sales of combined Hepatitis A and B vaccines which are often preferred for convenience. Public health strategies across the continent prioritize prevention in high-risk groups, including individuals with occupational exposure, chronic liver disease patients, and specific socially vulnerable populations identified through national epidemiological tracking. Pricing negotiation is a central feature of the European market, with large national health systems leveraging their collective purchasing power to secure favorable terms from manufacturers, impacting the overall market profitability structure.
Sustainability and accessibility are major considerations, leading to significant investments in specialized cold chain logistics tailored to pan-European distribution networks, crucial for maintaining vaccine potency across diverse geographical and climatic conditions. Furthermore, there is a regional emphasis on pharmaco-economic studies to justify the cost-effectiveness of mass vaccination programs, especially in countries where Hepatitis A is not endemic but sporadic outbreaks linked to contaminated food imports or travel occur periodically. This robust regulatory environment and emphasis on evidence-based procurement ensure sustained, high-quality demand for approved vaccines within the European Union and associated states, securing its position as a major global revenue contributor.
Asia Pacific (APAC): The Asia Pacific region is forecast to exhibit the fastest growth rate in the Hepatitis A Vaccine market, propelled by demographic factors, improving economic status, and the highest burden of endemicity globally. Countries like China, India, and Indonesia possess vast populations where Hepatitis A infection rates remain significantly higher compared to Western nations. This high endemicity initially provided natural immunity to older generations, but rapidly improving sanitation and hygiene standards are shifting the epidemiology, making younger cohorts susceptible and necessitating large-scale pediatric vaccination programs, a shift driving exponential growth in market volume.
Government initiatives across APAC, particularly in emerging economies, are focusing heavily on expanding universal immunization coverage. Strategic procurement by national health ministries, often supported by global alliances like Gavi, The Vaccine Alliance, characterizes the market access strategy. Local manufacturing capabilities, notably in India and China, are playing an increasingly crucial role, providing domestically produced, cost-effective vaccines that address the unique price sensitivity of these large markets. However, the logistical complexity of distributing vaccines across diverse geography, coupled with gaps in cold chain infrastructure in remote or rural areas, presents persistent challenges to achieving uniform coverage and requires innovative distribution solutions.
The competitive landscape in APAC is intensifying, with both global pharmaceutical giants and strong regional players vying for market share, often utilizing dual-tier pricing strategies tailored for government tenders versus private commercial sales. Consumer awareness regarding preventable diseases is rising, particularly among the expanding middle class, fueling private consumption through specialized clinics and pediatricians who recommend vaccination outside of mandatory government schedules. Investment in local R&D is also growing, targeting the development of locally tailored vaccine strains and enhanced formulations that can withstand regional distribution challenges, positioning APAC as not just a consumer market but also a vital center for future vaccine innovation and production capacity.
Latin America: The Latin American market for Hepatitis A vaccines presents a scenario of moderate growth, balanced between ongoing public health efforts and macroeconomic stability challenges in certain countries. HAV remains endemic across much of Central and South America, driving consistent demand for pediatric vaccination, often integrated into national immunization calendars. Countries such as Brazil, Mexico, and Argentina represent the largest markets, benefitting from established healthcare infrastructures and stable government procurement mechanisms that prioritize disease prevention initiatives and outbreak control measures, ensuring consistent, high-volume demand.
Market dynamics in this region are significantly shaped by regulatory harmonization attempts through regional bodies, although individual country approvals still dictate market entry. The reliance on imported vaccines is gradually being offset by regional production capacity, particularly for combination vaccines, aiming to achieve self-sufficiency and reduce reliance on international supply chains, which can be vulnerable to global shocks. The logistical challenge here centers on traversing vast distances and varied topographies, from dense urban centers to remote Amazonian or Andean communities, necessitating highly resilient and sometimes costly specialized transport and storage solutions to maintain efficacy.
Beyond routine immunization, the high incidence of travel within the continent and to North America fuels a steady demand in the private travel health sector. Targeted vaccination campaigns aimed at controlling localized outbreaks, often triggered by food or water contamination incidents, are also a crucial component of revenue generation. Pharmaceutical companies operating in Latin America must navigate complex national tendering processes and currency fluctuations, which impact procurement pricing and financial planning. Successful market penetration relies heavily on establishing strong relationships with national health authorities and demonstrating commitment to long-term supply, often including technology transfer or local packaging agreements.
Middle East and Africa (MEA): The Middle East and Africa represent a diverse market characterized by high heterogeneity in healthcare infrastructure, economic capacity, and epidemiological needs. In the Middle East, particularly the Gulf Cooperation Council (GCC) countries, high healthcare expenditure and strict governmental mandates lead to high penetration rates and stable, predictable demand, comparable to developed Western markets. Here, the adult segment is bolstered by large expatriate populations and high volumes of religious tourism, necessitating rigorous prophylactic vaccination protocols for large-scale gatherings.
Conversely, the African continent, facing severe resource constraints and the highest levels of HAV endemicity, relies heavily on global health initiatives and foreign aid (e.g., Gavi support, UNICEF procurement) for large-scale pediatric vaccination programs. While the need is immense, the purchasing power is low, making this region highly sensitive to pricing and dependent on donated or heavily subsidized doses. The primary challenge in Africa is not demand, but effective supply delivery: establishing and maintaining the critical cold chain across extremely challenging infrastructure and reaching remote populations amidst conflict or political instability. Innovative delivery models, including mobile clinics and optimized logistics planning, are vital for market success and public health impact.
Market growth in MEA is thus highly correlated with geopolitical stability, increased foreign direct investment in healthcare infrastructure, and the success of multilateral organizations in securing funding for mass immunization programs. Local production is nascent but growing, particularly in South Africa and Egypt, aiming to address regional supply needs. For pharmaceutical manufacturers, strategic success in Africa means focusing on robust, subsidized supply chains and developing formulations that are optimized for challenging field conditions, often prioritizing partnerships with local NGOs and government bodies over purely commercial sales, reflecting the public health imperative that dominates this regional market segment.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Hepatitis A Vaccine Market.- GlaxoSmithKline plc
- Sanofi
- Merck & Co., Inc.
- Emergent BioSolutions Inc.
- Johnson & Johnson (Janssen Global Services)
- Pfizer Inc.
- Bristol-Myers Squibb Company
- Serum Institute of India Pvt. Ltd.
- Bharat Biotech
- Sinovac Biotech Ltd.
- Shanghai Institute of Biological Products
- Takeda Pharmaceutical Company Limited
- Dynavax Technologies Corporation
- Mitsubishi Tanabe Pharma Corporation
- CSL Limited
- Bio Farma (Persero)
- SK Bioscience Co., Ltd.
- Crucell N.V. (Part of Johnson & Johnson)
- Berna Biotech (Part of Crucell/J&J)
- Valneva SE
Frequently Asked Questions
Analyze common user questions about the Hepatitis A Vaccine market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the projected Compound Annual Growth Rate (CAGR) for the Hepatitis A Vaccine Market?
The Hepatitis A Vaccine Market is projected to grow at a CAGR of 7.8% during the forecast period from 2026 to 2033, driven by expanded mandatory immunization programs and increasing international travel volume.
Which geographical region dominates the global Hepatitis A Vaccine market?
North America currently holds a dominant revenue share due to high healthcare expenditure, established universal pediatric immunization schedules, and significant demand from high-risk adult populations and international travelers.
What key factors are restraining the growth of the Hepatitis A Vaccine market, especially in developing regions?
Primary restraints include the challenges associated with maintaining the strict cold chain requirements (2°C to 8°C) necessary for vaccine stability during distribution, coupled with high procurement costs relative to the healthcare budgets of low- and middle-income countries.
What type of vaccine formulation currently holds the largest market share?
Inactivated Hepatitis A vaccines (whole-virus, formalin-inactivated) hold the largest market share. This dominance is attributed to their proven high efficacy, long-term safety record, and widespread acceptance by regulatory bodies worldwide for routine immunization.
How is AI expected to influence future Hepatitis A vaccine supply and distribution?
AI is anticipated to significantly influence the market by improving predictive epidemiology to forecast outbreaks, optimizing complex supply chain and cold chain logistics, and streamlining manufacturing processes to increase yield and reduce production costs.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager