Extracorporeal Circulation Supporting Intubation Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 434672 | Date : Dec, 2025 | Pages : 258 | Region : Global | Publisher : MRU
Extracorporeal Circulation Supporting Intubation Market Size
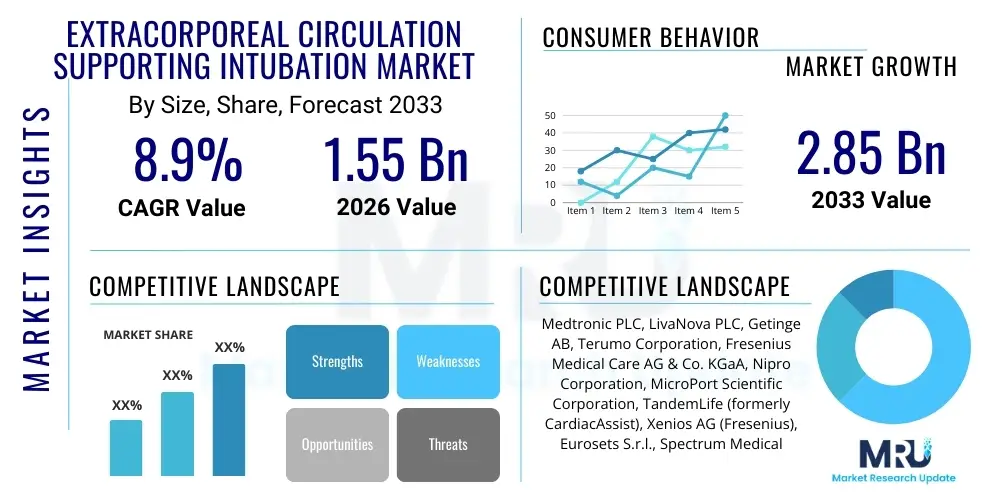
The Extracorporeal Circulation Supporting Intubation Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.9% between 2026 and 2033. The market is estimated at USD 1.55 Billion in 2026 and is projected to reach USD 2.85 Billion by the end of the forecast period in 2033. This robust growth trajectory is primarily fueled by the increasing prevalence of acute respiratory distress syndrome (ARDS), the rising necessity for advanced temporary circulatory support during complex interventional procedures, and the continuous technological evolution in portable and miniaturized ECMO systems designed specifically for emergency and critical care settings. The strategic expansion of ECMO centers globally, coupled with enhanced training protocols for critical care specialists, further contributes to the expanding application base of extracorporeal circulation techniques.
The market expansion is also significantly influenced by the growing awareness and adoption of ECMO (Extracorporeal Membrane Oxygenation) as a viable bridge to recovery or transplantation, especially in cases where conventional mechanical ventilation proves insufficient or detrimental to the patient’s outcome. Technological advancements focusing on biocompatibility, reduced priming volumes, and integration with advanced monitoring systems are improving safety profiles and reducing complication rates associated with prolonged extracorporeal support. Furthermore, the development of specialized cannulas and circuits optimized for rapid deployment and ease of use in airway management and intubation scenarios is driving the shift from traditional approaches to supported intubation.
Extracorporeal Circulation Supporting Intubation Market introduction
The Extracorporeal Circulation Supporting Intubation Market encompasses the medical devices, consumables, and systems utilized to provide temporary circulatory or respiratory support during the process of endotracheal intubation, particularly in patients experiencing severe hemodynamic instability or acute respiratory failure. These systems, predominantly based on ECMO technology (both Veno-Venous (VV) and Veno-Arterial (VA) ECMO), stabilize the patient's oxygenation and perfusion status, thereby converting a high-risk intubation scenario into a controlled procedure. The critical importance of these devices lies in their ability to mitigate the profound hypoxemia and cardiac arrest risk often associated with securing the airway in critically ill populations, ensuring patient survival and minimizing secondary brain injury.
The product portfolio includes high-performance centrifugal pumps, specialized oxygenators (membrane lungs), precise heat exchangers, sophisticated monitoring consoles, and sterile disposable tubing kits and cannulas. Major applications span adult and pediatric critical care units, emergency departments, operating rooms, and dedicated cardiac and pulmonary intensive care units. Key benefits include drastically improved oxygen reserve during intubation attempts, reduction in procedural stress on the cardiovascular system, enhanced safety for both patient and clinician, and the facilitation of complex airway management in anatomically difficult cases. Driving factors include the increasing global burden of chronic obstructive pulmonary disease (COPD) and pneumonia, advancements in minimally invasive cannulation techniques, and robust reimbursement policies supporting complex critical care interventions.
Extracorporeal Circulation Supporting Intubation Market Executive Summary
The Extracorporeal Circulation Supporting Intubation Market is defined by dynamic business trends, marked by substantial investments in portable ECMO systems and strategic collaborations between medical device manufacturers and specialized hospitals to establish dedicated Mobile ECMO Teams. Business success is increasingly reliant on developing integrated systems that offer automation, reduced complexity, and enhanced connectivity for remote monitoring, addressing the skilled personnel shortage challenge prevalent in many healthcare systems. The market is transitioning towards disposable, single-use components that ensure sterility and minimize cross-contamination risks, driving innovation in material science and biocompatibility. Furthermore, the integration of simulation training platforms using market devices is becoming a critical business offering, ensuring widespread and competent clinical adoption.
Regionally, North America maintains market dominance due to high healthcare expenditure, the presence of major key players, and established guidelines supporting early implementation of ECMO for respiratory failure and intubation support. However, the Asia Pacific (APAC) region is demonstrating the highest growth potential, spurred by improving healthcare infrastructure in developing economies like China and India, increasing medical tourism for complex procedures, and rising government initiatives focused on enhancing critical care capabilities. Europe represents a mature but stable market, focusing on optimizing existing clinical protocols and standardizing device utilization across various national health services, often prioritizing cost-effectiveness and long-term device performance.
Segment trends highlight the critical role of the Oxygenators segment, which commands a significant market share due to the necessity of frequent replacement and advancements in polymethylpentene (PMP) fiber technology, which offers superior gas exchange efficiency and durability. Application-wise, the Acute Respiratory Distress Syndrome (ARDS) segment remains the primary revenue generator, given the severe respiratory compromise necessitating supported intubation. The shift towards miniaturized and highly integrated ECMO circuits (often termed 'mini-ECMO') is a defining trend across all segments, promising broader applicability in non-traditional settings such as pre-hospital care and rapid trauma management, fundamentally changing the logistical footprint of extracorporeal support.
AI Impact Analysis on Extracorporeal Circulation Supporting Intubation Market
User inquiries regarding the impact of Artificial Intelligence (AI) on the Extracorporeal Circulation Supporting Intubation Market frequently revolve around three core themes: improving patient selection accuracy for ECMO support, optimizing device parameters in real-time during the procedure, and predicting complications such as bleeding or circuit failure. Users express high expectations for AI models capable of analyzing complex physiological data (blood gas, hemodynamics, ventilation settings) instantaneously to provide predictive analytics on intubation outcomes and necessary pre-procedural stabilization measures. A common concern is the trustworthiness and validation of AI algorithms in high-stakes, time-critical environments like the emergency department, where rapid decision-making is paramount. Expectations are also high for AI-driven training simulations that can mimic realistic patient deterioration scenarios specific to airway management under extracorporeal support, enhancing clinician proficiency.
AI’s initial influence is manifested through sophisticated predictive monitoring systems that analyze perfusion and oxygenation trends, alerting clinicians to subtle deviations indicating impending patient collapse before intubation is attempted. Advanced algorithms are being developed to personalize ventilation strategies following intubation while the patient remains on ECMO, aiming to minimize ventilator-induced lung injury (VILI). Furthermore, AI is crucial in streamlining complex logistical processes, such as managing the inventory of specialized components and optimizing the staffing of perfusionists and ECMO specialists across hospital networks. This transition towards augmented clinical decision-making is expected to standardize best practices and significantly reduce variability in patient care quality, especially in institutions with lower ECMO case volumes.
The long-term impact of AI involves the integration of machine learning into the ECMO console itself, allowing for automated feedback loops that adjust flow rates, sweep gas settings, and temperature based on continuous input from patient physiological sensors. This level of automation reduces the cognitive load on critical care staff during highly stressful intubation procedures. Furthermore, AI is playing a growing role in post-procedural data analysis, identifying patterns that correlate specific intubation approaches under ECMO support with long-term patient recovery and discharge outcomes. This data-driven feedback loop is essential for continuous quality improvement and the refinement of existing clinical practice guidelines related to supported airway management.
- AI-driven predictive analytics for identifying high-risk intubation candidates needing pre-emptive ECMO.
- Real-time automated optimization of pump flow and sweep gas during the intubation procedure to maintain physiological targets.
- Enhanced simulation and training modules using AI to create realistic, dynamic patient scenarios under extracorporeal support.
- Machine learning algorithms analyzing hemodynamic stability to predict and prevent procedural complications.
- Optimization of circuit monitoring, including early detection of thrombus formation or membrane lung failure through sensor data analysis.
DRO & Impact Forces Of Extracorporeal Circulation Supporting Intubation Market
The Extracorporeal Circulation Supporting Intubation Market is shaped by a confluence of powerful drivers, stringent restraints, and significant strategic opportunities, collectively defining the Impact Forces. Key drivers include the escalating global incidence of acute and chronic cardiopulmonary diseases, demanding advanced life support technologies. Furthermore, the demonstrated success and increasing adoption of ECMO during the COVID-19 pandemic significantly accelerated the acceptance of extracorporeal support in critical care, cementing its role in complex airway management. Technological miniaturization and improved device portability are making these systems accessible beyond highly specialized cardiac centers, extending their use to rapid response teams and community hospitals. The market's dynamism is rooted in the clinical necessity of these devices to ensure safe intubation in the sickest patient populations.
However, substantial restraints impede unchecked growth. The primary barrier is the high upfront capital cost associated with ECMO equipment and the recurrent expenditure on specialized disposable circuits. Furthermore, the clinical application of these technologies requires highly specialized, multidisciplinary teams, including trained perfusionists, critical care physicians, and nurses; the scarcity of this highly skilled workforce globally restricts broader implementation, particularly in lower-resource settings. Associated clinical risks, such as bleeding complications, hemolysis, and neurological events linked to prolonged extracorporeal support, necessitate careful patient selection and continuous vigilance, occasionally tempering enthusiasm for widespread use. Stringent regulatory approval pathways in various jurisdictions also pose a time-consuming and costly hurdle for manufacturers introducing new, innovative devices.
Opportunities for market growth are extensive and promising. Strategic areas include developing cost-effective, regionally optimized ECMO systems suitable for emerging economies. The integration of advanced sensor technology for non-invasive monitoring and real-time data feedback offers significant avenues for product differentiation. Moreover, expanding the application scope to include prophylactic intubation support in high-risk patients undergoing non-cardiac surgery or complex endoscopic procedures represents a substantial untapped potential. The industry is also focused on developing simplified, pre-primed circuits that can be deployed rapidly by less specialized personnel in emergency settings, effectively democratizing access to supported intubation techniques and mitigating the constraint related to specialized staff availability.
- Drivers: Increasing prevalence of ARDS and severe respiratory failure; growing adoption of ECMO as a standard of care; technological advancements in portable and miniaturized devices; strong supportive reimbursement for critical care procedures.
- Restraints: High initial investment and operational costs; shortage of skilled personnel (perfusionists and specialized nurses); inherent risks of anticoagulation and bleeding complications; complex logistical requirements for device maintenance and sterilization.
- Opportunities: Expansion into pre-hospital and mobile ECMO services; development of fully integrated, automated systems; market penetration in underserved emerging economies; research into non-anticoagulant ECMO coatings to reduce bleeding risks.
- Impact Forces: High clinical efficacy and undeniable life-saving potential (high impact force) counterbalanced by significant human resource constraints (moderating force), driving innovation towards automation and simplicity.
Segmentation Analysis
The Extracorporeal Circulation Supporting Intubation Market is meticulously segmented based on components, application areas, and end-users, reflecting the diverse clinical needs and technological complexities inherent in providing supported critical care. Segmentation by component is crucial as it dictates the cost structure and technological differentiation within the market, covering essential disposables like oxygenators and cannulas, which generate high recurring revenue, alongside durable equipment like pumps and control units. The application segmentation delineates the primary clinical scenarios where this support is utilized, with pulmonary support (VV-ECMO) dominating the volume, while cardiac support (VA-ECMO) requires more complex, specialized setups. This granular breakdown enables manufacturers to tailor product development and marketing strategies to specific clinical environments and regulatory requirements.
The segmentation by end-user reflects the primary adoption centers, primarily large academic hospitals and specialized critical care units which possess the necessary infrastructure and expertise for maintaining ECMO programs. A growing trend is the emergence of smaller critical access hospitals and specialized ambulatory surgical centers (ASCs) beginning to adopt limited scope ECMO services, primarily through mobile or consortium agreements, driving the demand for user-friendly and rapidly deployable systems. Understanding these segments is vital for analyzing competitive dynamics, identifying high-growth niches, and forecasting future technological requirements. For instance, the high mortality associated with severe respiratory failure continues to prioritize investments in enhanced oxygenation efficiency across all components.
- By Component:
- Pumps (Centrifugal Pumps, Roller Pumps)
- Oxygenators/Membrane Lungs (Polypropylene, PMP)
- Cannulas and Catheters (Veno-Venous, Veno-Arterial)
- Tubing and Circuit Kits (Disposable, Integrated)
- Monitoring and Control Systems (Flow Sensors, Pressure Monitors)
- By Application:
- Acute Respiratory Distress Syndrome (ARDS)
- Cardiogenic Shock
- Pulmonary Embolism
- Bridge-to-Transplant/Recovery
- Cardiac Arrest (E-CPR)
- By End-User:
- Hospitals (Academic Medical Centers, Specialty Hospitals)
- Ambulatory Surgical Centers (Limited Scope)
- Specialized Critical Care Units
- By Patient Age Group:
- Adult
- Pediatric and Neonatal
Value Chain Analysis For Extracorporeal Circulation Supporting Intubation Market
The value chain for the Extracorporeal Circulation Supporting Intubation Market is intricate, starting with highly specialized upstream suppliers focusing on biomaterials, advanced plastics, and sophisticated sensor technology. Upstream analysis involves raw material providers for polymethylpentene fibers (used in oxygenators) and biocompatible polymers for tubing and cannulas. The quality and purity of these raw materials are critical, as they directly influence device performance, safety, and regulatory compliance. Suppliers in this segment face high barriers to entry due to the necessity for specialized certifications and long-term contracts with device manufacturers, emphasizing precision engineering and strict quality control.
The middle segment of the value chain is dominated by the core ECMO system manufacturers who engage in R&D, design, assembly, and rigorous testing of integrated pump and oxygenation systems. This manufacturing process involves complex integration of mechanical, fluidic, and electronic components. Downstream analysis focuses on the distribution channels and end-user facilities. Products move through a combination of direct sales channels, particularly for large capital equipment and complex integrated systems requiring extensive technical support and installation, and indirect distribution through specialized medical distributors and Group Purchasing Organizations (GPOs) for high-volume consumables like tubing and cannulas.
Direct sales are prevalent for initial capital purchases and in regions demanding close manufacturer interaction for training and maintenance. Indirect channels leverage the extensive logistical networks of distributors to ensure rapid supply of disposable components to critical care units worldwide. The end of the chain involves the specialized critical care providers—hospitals and medical centers—which utilize the equipment. Effective value delivery relies heavily on post-sale technical support, timely supply of proprietary consumables, and continuous clinical education provided by the manufacturer or authorized distributors to ensure optimal and safe use during critical intubation procedures.
Extracorporeal Circulation Supporting Intubation Market Potential Customers
The primary potential customers and end-users of extracorporeal circulation systems used to support intubation are large, tertiary-level academic medical centers. These institutions manage the highest acuity patients, possess dedicated cardiac and pulmonary intensive care units (ICUs), and maintain established ECMO centers of excellence. Their patient population often includes those with severe refractory respiratory failure, cardiogenic shock, or complex co-morbidities requiring highly skilled management during airway procedures. These centers are responsible for the highest volume of procedures and are typically early adopters of new technological advancements, driving the demand for premium, high-performance systems and integrated monitoring capabilities.
Secondary, but rapidly growing, customer segments include regional specialized critical care units and medium-sized community hospitals that have begun implementing limited or decentralized ECMO programs. While these facilities may not handle the sheer volume of a large academic center, the increasing recognition of ECMO as a necessary life-support modality mandates its availability, especially for emergency supported intubations. This customer base seeks solutions that are highly reliable, user-friendly, and require minimal technical maintenance, often favoring portable and modular systems that can be rapidly deployed in various departments, including the emergency room and catheterization laboratory.
Furthermore, mobile critical care transport teams and specialized military medical units represent niche but significant potential customers. These teams require ultra-portable, ruggedized ECMO systems designed for rapid stabilization and sustained support during patient transfer, often performing supported intubation in uncontrolled environments. Their purchasing criteria prioritize durability, low weight, battery life, and compatibility with various air and ground transport modes. Procurement strategies in this segment emphasize bundled purchases that include extensive training and comprehensive maintenance contracts suitable for remote operations.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 1.55 Billion |
| Market Forecast in 2033 | USD 2.85 Billion |
| Growth Rate | 8.9% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Medtronic PLC, LivaNova PLC, Getinge AB, Terumo Corporation, Fresenius Medical Care AG & Co. KGaA, Nipro Corporation, MicroPort Scientific Corporation, TandemLife (formerly CardiacAssist), Xenios AG (Fresenius), Eurosets S.r.l., Spectrum Medical Ltd., ALung Technologies, Inc., Sorin Group (LivaNova), MAQUET (Getinge), Braile Biomédica. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Extracorporeal Circulation Supporting Intubation Market Key Technology Landscape
The technological landscape of the Extracorporeal Circulation Supporting Intubation Market is dominated by continuous innovation aimed at reducing invasiveness, enhancing device longevity, and improving the logistical feasibility of ECMO deployment. Key technologies include the miniaturization of centrifugal pumps, which are now often magnetically driven and designed for low shear stress, minimizing blood cell damage (hemolysis). This move away from older, bulkier roller pumps is crucial for mobile applications. Furthermore, advancements in oxygenator design, particularly the adoption of Polymethylpentene (PMP) fiber technology, have drastically improved gas exchange efficiency and reduced plasma leakage, extending the duration for which a single oxygenator can be safely used, which is vital for patients requiring prolonged support prior to intubation or recovery.
Another significant technological focus is on biocompatibility and surface coatings. Manufacturers are investing heavily in heparin-bonded surfaces and other novel non-thrombogenic coatings for tubing and cannulas. The goal is to minimize the systemic inflammatory response and the need for high-dose anticoagulation, thereby reducing the risk of major bleeding complications—a leading cause of morbidity and mortality associated with ECMO support. Integrated monitoring systems represent the third pillar of technology, incorporating advanced sensors for real-time measurement of parameters such as venous oxygen saturation, activated clotting time (ACT), and circuit pressure differentials. These sophisticated systems facilitate predictive maintenance alerts and allow clinicians to make rapid, data-driven decisions during the critical intubation period.
The trend towards integrated, consolidated consoles that combine the pump, oxygenator, and control unit into a single, user-friendly device represents a major leap forward for supporting intubation in non-traditional settings. These highly integrated systems often feature intuitive interfaces and automated priming capabilities, significantly reducing setup time and the reliance on highly trained perfusionists for deployment. The emerging technology of computational fluid dynamics (CFD) modeling is also being applied to optimize cannula tip design, ensuring efficient blood drainage and return with minimal recirculation, thereby maximizing the therapeutic effect of the extracorporeal circuit during supported airway procedures.
Regional Highlights
North America: North America, comprising the United States and Canada, holds the dominant share of the Extracorporeal Circulation Supporting Intubation Market. This market leadership is attributed to several factors: exceptionally high healthcare spending, particularly in critical care and advanced life support; the presence of numerous large academic medical centers with mature, high-volume ECMO programs; and favorable, well-established reimbursement policies for complex procedures like ECMO. The U.S. market is characterized by rapid adoption of the latest technological innovations, driven by competitive pressures among hospitals to offer the highest level of critical care services. The region also benefits from robust clinical research activities and standardized training protocols developed by organizations like the Extracorporeal Life Support Organization (ELSO), ensuring high standards of care and increasing the confidence in utilizing ECMO for supported intubation in high-risk patients. The demand is particularly strong for highly advanced, portable VA-ECMO systems used in cardiogenic shock patients needing rapid airway securement.
Europe: The European market represents a mature but technologically sophisticated landscape, characterized by centralized healthcare systems and a focus on cost-effectiveness and standardization. Western European countries, including Germany, France, and the UK, are significant contributors to market revenue, driven by aging populations prone to cardiopulmonary diseases and established national guidelines for managing severe respiratory failure. The European market emphasizes long-term performance, biocompatibility, and integration capabilities of ECMO circuits, seeking solutions that can safely provide support for extended durations post-intubation. Regulatory procedures, particularly the MDR (Medical Device Regulation), are stringent, which influences product design towards proven safety and clinical utility. Nordic countries are also pioneering the use of mobile ECMO units, contributing to the demand for compact and efficient devices that can be rapidly transported between hospitals.
Asia Pacific (APAC): The APAC region is forecast to be the fastest-growing market, presenting immense opportunities due to massive improvements in healthcare infrastructure and increasing public and private investments in critical care facilities across China, India, Japan, and South Korea. While Japan and South Korea boast highly advanced medical technologies and adoption rates comparable to the West, the emerging economies of China and India are driving volume growth. The rapid urbanization, coupled with environmental factors contributing to respiratory illnesses, dramatically increases the patient pool requiring sophisticated respiratory support. Challenges remain regarding training and affordability, leading to strong demand for locally manufactured, cost-effective ECMO solutions and increased interest in international partnerships to transfer clinical expertise and expand regional ECMO center networks. Government initiatives focused on combating pandemic preparedness also fuel the purchasing of life-support equipment.
- North America: Market leader; driven by high critical care expenditure, advanced technological adoption, and robust ECMO training infrastructure; high demand for portable VA-ECMO systems.
- Europe: Mature market focusing on standardization, cost-efficiency, and adherence to rigorous regulatory standards (MDR); strong adoption of optimized PMP oxygenators.
- Asia Pacific (APAC): Fastest-growing region; expansion fueled by infrastructure development, rising respiratory disease prevalence, and significant demand for cost-effective, high-volume consumables, particularly in China and India.
- Latin America & MEA (LAMEA): Emerging regions; growth constrained by economic instability and variable healthcare access, but driven by centralized critical care centers in major cities and increasing utilization of philanthropic and international aid programs to acquire essential life support technologies.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Extracorporeal Circulation Supporting Intubation Market.- Medtronic PLC
- LivaNova PLC
- Getinge AB
- Terumo Corporation
- Fresenius Medical Care AG & Co. KGaA
- Nipro Corporation
- MicroPort Scientific Corporation
- TandemLife (formerly CardiacAssist)
- Xenios AG (A Fresenius Medical Care Company)
- Eurosets S.r.l.
- Spectrum Medical Ltd.
- ALung Technologies, Inc.
- Braile Biomédica
- Lifeline Scientific, Inc.
- Abbott Laboratories
- Teleflex Incorporated
- Edwards Lifesciences Corporation
- Becton, Dickinson and Company (BD)
- Intersurgical Ltd.
- CytoSorbents Corporation
Frequently Asked Questions
Analyze common user questions about the Extracorporeal Circulation Supporting Intubation market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is Extracorporeal Circulation Supporting Intubation and why is it necessary?
Extracorporeal Circulation Supporting Intubation involves using ECMO (Extracorporeal Membrane Oxygenation) systems to provide temporary life support during the insertion of a breathing tube (intubation) in critically ill patients. It is necessary because these patients often lack the physiological reserves to tolerate the momentary loss of oxygenation during the intubation attempt, preventing cardiac arrest and severe hypoxemia.
What are the primary technological innovations driving the ECMO market?
The market is primarily driven by miniaturization and portability of ECMO consoles, advancements in highly biocompatible PMP oxygenator technology for extended use, and the development of integrated monitoring systems that provide real-time data for automated flow management, enhancing patient safety during critical procedures.
Which market segment holds the highest growth potential in the forecast period?
The Asia Pacific (APAC) region, specifically the emerging economies within it, exhibits the highest growth potential due to rapid investment in critical care infrastructure, increasing prevalence of respiratory diseases, and the necessity for scalable, cost-effective ECMO solutions to meet high population demand.
What clinical complications are associated with Extracorporeal Circulation and how are manufacturers mitigating them?
Major clinical complications include bleeding, thrombus formation (clotting), and hemolysis. Manufacturers are mitigating these risks by developing advanced non-thrombogenic surface coatings for circuits and cannulas (e.g., heparin bonding), optimizing fluid dynamics within devices to reduce shear stress, and designing integrated sensors for early detection of circuit degradation.
How does the shortage of skilled personnel impact the adoption of these systems?
The global shortage of trained perfusionists and critical care specialists acts as a significant restraint, limiting the ability of many hospitals to establish or expand ECMO programs. Manufacturers are addressing this by developing highly automated, user-friendly consoles with simpler operational interfaces and providing comprehensive simulation-based training programs to rapidly increase the competency of existing clinical staff.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
