Germ Free Mice Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 435155 | Date : Dec, 2025 | Pages : 258 | Region : Global | Publisher : MRU
Germ Free Mice Market Size
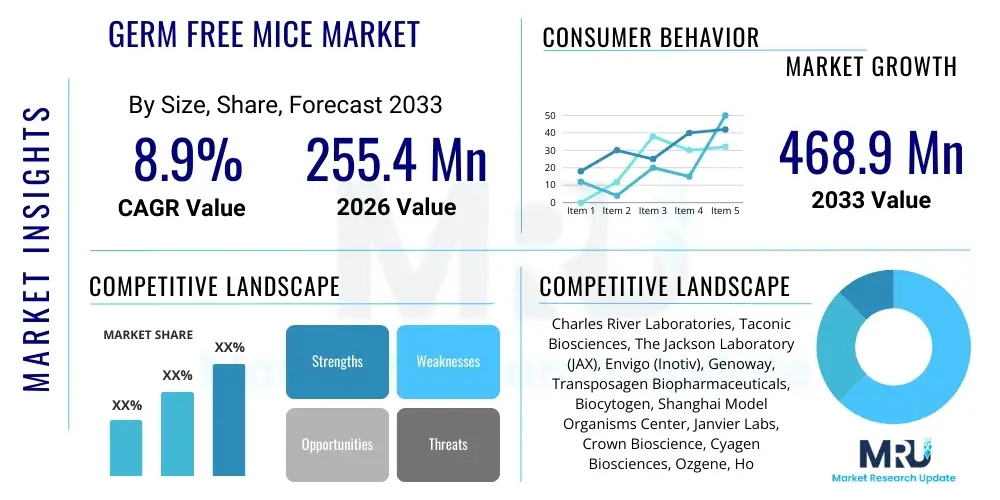
The Germ Free Mice Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.9% between 2026 and 2033. The market is estimated at $255.4 Million USD in 2026 and is projected to reach $468.9 Million USD by the end of the forecast period in 2033.
Germ Free Mice Market introduction
The Germ Free Mice Market, often referred to as the Gnotobiotic Mice Market, involves the development, production, and supply of laboratory mice that are entirely devoid of detectable microorganisms, including bacteria, fungi, viruses, and parasites. These specialized models are maintained in highly controlled, sterile environments, typically within rigid or flexible isolators, ensuring their germ-free (axenic) status is preserved for the duration of research protocols. The primary purpose of these models is to facilitate research requiring a baseline state completely free of microbial influence, making them indispensable tools for studying the complex roles of specific microbial populations, host-microbe interactions, immunity, and metabolism.
Germ Free Mice are pivotal across several major scientific disciplines, particularly immunology and gastroenterology, where they allow researchers to introduce known microbial communities (defined flora) to study specific physiological or pathological outcomes—a process known as colonization. Major applications include unraveling the etiology of inflammatory bowel disease, assessing the efficacy and safety of novel probiotics or fecal microbial transplantation (FMT) techniques, and investigating how the microbiome modulates response to oncology treatments, such as immunotherapy. The unique physiological state of these animals offers unparalleled clarity in complex biological systems.
The demand for these models is significantly driven by the explosive growth in microbiome research globally and the increasing acknowledgment within pharmaceutical and biotechnology sectors that the gut flora profoundly impacts drug efficacy and toxicity. Benefits derived from using germ-free models include precision in experimental design, the ability to isolate and attribute phenotypic changes directly to specific introduced microbes, and enhanced rigor in preclinical testing. Key driving factors include increasing R&D investments in personalized medicine, rising prevalence of chronic diseases linked to microbial dysbiosis, and technological advancements in gnotobiotic housing and genetic manipulation techniques.
Germ Free Mice Market Executive Summary
The global Germ Free Mice market is characterized by robust growth, primarily fueled by pharmaceutical industry investments in the gut-brain axis and oncology research. Business trends show a strategic shift toward generating more complex and customizable gnotobiotic models, including humanized microbiota mice (HMM), which bridge the gap between basic research and clinical applications. Leading contract research organizations (CROs) are increasingly integrating germ-free animal services into their preclinical portfolios, offering specialized study designs and high-throughput screening capabilities, thereby democratizing access to these resource-intensive models for smaller biotech firms. Supply chain resilience, particularly the management of specialized isolator equipment and the maintenance of highly skilled veterinary staff, remains a critical operational focus for key market players.
Regionally, North America continues to dominate the market, driven by substantial government and private sector funding directed towards life sciences research, particularly within elite academic institutions and large pharmaceutical hubs. Asia Pacific, specifically China and Japan, is projected to exhibit the highest growth rate due to rapidly expanding domestic biotechnology sectors, increased government emphasis on infectious disease research following recent global health crises, and significant investments in establishing high-containment, gnotobiotic facilities. European growth is steady, supported by collaborative research networks focusing on immunology and metabolic disorders, often backed by EU-level research grants emphasizing standardization and harmonization of animal models.
Segment trends highlight the dominance of the Gut Microbiome Research application segment, reflecting the massive volume of studies aimed at understanding microbial contributions to health and disease. In terms of product type, genetically modified germ-free models, such as Knockout and Transgenic mice, are experiencing accelerated demand over standard Inbred strains, as researchers require models that combine microbial sterility with specific genetic deficiencies or enhancements. End-user analysis shows that Pharmaceutical and Biotechnology companies, driven by the need for regulatory-compliant preclinical data for novel therapeutics targeting the microbiome, represent the most lucrative customer segment, demanding large cohorts of highly standardized models within tight timelines.
AI Impact Analysis on Germ Free Mice Market
Common user inquiries regarding AI’s impact on the Germ Free Mice Market revolve primarily around optimizing experimental design, accelerating the analysis of vast omics data generated from gnotobiotic studies, and improving the efficiency of colony management. Users are concerned about how AI can handle the complexity of host-microbe interactions—specifically, correlating changes in the microbial community (metagenomics/metabolomics) with complex host phenotypes (transcriptomics/proteomics) observed in axenic or colonized mice. Key themes include expectations that AI will significantly reduce the time required to derive meaningful, actionable insights from high-dimensional datasets and streamline the logistical challenges associated with maintaining stringent barrier controls and predicting potential breaches in germ-free status. Users also anticipate AI algorithms will assist in designing optimal microbial community introduction protocols, moving beyond trial-and-error methodologies.
AI adoption is poised to revolutionize the interpretive phase of gnotobiotic research. The studies generate enormous datasets related to host physiology, metabolomics, and microbial genetics, which are often too complex for traditional statistical methods to fully decouple. Machine learning models, particularly deep learning networks, are adept at identifying subtle, non-linear correlations between specific microbial strains, their metabolites, and host immune responses or disease progression, leading to faster hypothesis generation and validation. This analytical power enhances the value proposition of Germ Free Mice studies by extracting maximum biological relevance from each resource-intensive experiment.
Furthermore, AI-driven predictive maintenance and monitoring systems are transforming the operational aspects of gnotobiotic housing. These systems utilize continuous sensor data (temperature, humidity, pressure differentials) within isolators and barrier facilities, applying predictive analytics to detect minute deviations that could compromise the germ-free status. By enabling proactive intervention, AI minimizes the risk of colony contamination, which is catastrophic in terms of research timelines and financial expenditure. This application of AI ensures higher reliability and integrity of the most precious research assets, translating directly into enhanced confidence in preclinical results.
- AI optimizes complex omics data interpretation (metagenomics, metabolomics, transcriptomics) from colonized models.
- Machine learning identifies novel, subtle host-microbe interaction patterns critical for therapeutic development.
- Predictive analytics enhance gnotobiotic barrier integrity and isolator maintenance, reducing contamination risks.
- AI assists in rational design of defined microbial communities (consortia) for colonization studies.
- Automation and robotic integration, guided by AI, streamline high-throughput phenotyping in sterile environments.
DRO & Impact Forces Of Germ Free Mice Market
The Germ Free Mice market is propelled by significant drivers, primarily the burgeoning investment in basic and translational research focused on the human microbiome and its role in chronic diseases, particularly autoimmunity, neurology (gut-brain axis), and metabolism. The increasing understanding that the efficacy of many modern drugs, especially checkpoint inhibitors in oncology, is fundamentally linked to the composition of the host microbiota mandates the use of germ-free or gnotobiotic models for mechanism elucidation and preclinical validation. This scientific imperative is the strongest driver. Furthermore, advancements in genetic engineering technologies (like CRISPR/Cas9) allow for the rapid creation of genetically complex germ-free models, accelerating the pace of targeted drug discovery.
However, significant restraints temper market growth. The extremely high operational costs associated with establishing and maintaining stringent germ-free barrier facilities, including specialized isolator equipment, sterilized feeds, and highly trained personnel, limit the number of institutions capable of producing these models. Ethical constraints and public scrutiny regarding animal usage in research, particularly for complex models, continue to pose regulatory hurdles. Additionally, the inherent logistical challenge of transporting and handling these delicate, highly sensitive animals without compromising their axenic status creates supply chain bottlenecks, especially for global distribution.
Opportunities for market expansion are vast, centering on the development of humanized microbiome mouse models (HMMs) for personalized medicine studies, where a patient's own microbiota is transferred into a germ-free mouse. This enables precision preclinical testing. The rising demand from Contract Research Organizations (CROs) for specialized gnotobiotic services presents an immediate growth avenue, allowing academic and smaller biotech companies to outsource complex studies. The primary impact force accelerating market trajectory is the increasing linkage of microbial factors to CNS disorders and autoimmune conditions, pushing research expenditures toward gnotobiotic tools as essential platforms for novel therapeutic breakthroughs.
Segmentation Analysis
The Germ Free Mice market segmentation provides granular insight into the specific demands driving research and commercial activities. This market is intrinsically diversified by the research applications requiring these highly controlled models, the specific genetic background or modification of the mouse used, and the nature of the institutions utilizing them. Understanding these segments is crucial for suppliers to tailor their offerings, such as developing specific protocols for microbiome transfer tailored to oncology studies or optimizing supply chain logistics for high-volume academic orders. The complexity and resource intensity of gnotobiotic facilities mean that segmentation analysis often reflects the prioritization of research funding in specific disease areas globally.
The application segmentation reveals the primary areas where germ-free research is concentrated. While immunology and infectious diseases have historically been major users, the Gut Microbiome Research segment has witnessed exponential growth due to groundbreaking discoveries linking the gut flora to virtually every systemic biological process. Product type segmentation demonstrates a clear shift toward advanced genetic models. Standard inbred strains serve as foundational controls, but sophisticated research increasingly necessitates genetically engineered mice (transgenic, knockout, or knock-in models) maintained under germ-free conditions to isolate the combined effect of specific genes and controlled microbiota.
The end-user segment clearly delineates commercial drivers versus institutional needs. Pharmaceutical and Biotechnology Companies often require high volumes of customized models under strict quality controls for drug development and regulatory submissions, representing the highest revenue potential. Academic and Research Institutes constitute a high-volume, lower-margin segment focused primarily on basic mechanistic studies. CROs act as critical intermediaries, consolidating demand from multiple smaller entities and driving standardization and efficiency in gnotobiotic services, thereby facilitating market access for sophisticated research techniques.
- By Application:
- Gut Microbiome Research
- Infectious Disease Research
- Oncology
- Immunology and Autoimmune Disorders
- Metabolic Disorders (e.g., Obesity, Diabetes)
- Drug Discovery and Development
- By Product Type:
- Inbred Mice (e.g., C57BL/6J, BALBc)
- Outbred Mice
- Knockout Mice
- Transgenic Mice
- By End User:
- Pharmaceutical and Biotechnology Companies
- Academic and Research Institutes
- Contract Research Organizations (CROs)
Value Chain Analysis For Germ Free Mice Market
The value chain for the Germ Free Mice market is highly specialized and resource-intensive, beginning with the upstream sourcing and maintenance of foundation breeding stock, which must be rigorously characterized and maintained under strict Specific Pathogen Free (SPF) status before being moved into axenic isolators. Upstream activities are dominated by specialized commercial suppliers and large academic centers, requiring significant investment in facility infrastructure, including specialized HVAC systems, positive pressure environments, and barrier personnel training. The integrity of the isolator equipment, sterilization protocols for all inputs (food, water, bedding, tools), and the application of advanced genetic engineering techniques (CRISPR/Cas9) are critical quality checkpoints in this initial stage.
The core midstream process involves the meticulous breeding, colony expansion, and maintenance of the germ-free status throughout the lifespan of the animal. This includes continuous microbiological monitoring, often via sophisticated molecular diagnostics, to confirm the absence of contaminants. Direct distribution channels are prevalent due to the need for specialized logistics; major providers typically ship animals directly to the end-user’s research facility using highly secure, sterile shipping containers. Indirect distribution through third-party distributors is less common but used in regions lacking direct supplier presence, requiring rigorous adherence to cold chain and sterility protocols by the logistics partner.
Downstream analysis focuses on the end-users: pharmaceutical firms, academic labs, and CROs. Once the mice are delivered, the downstream value includes the research protocols themselves, such as controlled colonization (gnotobiotic studies), phenotypic analysis, and generation of complex data (omics). The high cost and complexity of the models mean that the value realization downstream is heavily dependent on the quality and rigor of the experimental output. The increasing demand for comprehensive services, including specialized study design consultancy and outsourced gnotobiotic execution provided by CROs, signifies a move toward a service-oriented downstream model, maximizing the utility of these valuable research assets.
Germ Free Mice Market Potential Customers
The primary potential customers and end-users of Germ Free Mice models span the entire spectrum of biomedical research, ranging from fundamental scientific inquiry to targeted preclinical drug validation. Pharmaceutical and large biotechnology companies represent the most commercially valuable segment, driven by the intense pressure to develop novel therapeutics targeting microbiome-related pathways, including oncology agents, metabolic disease treatments, and novel antibiotics. These organizations require large, standardized cohorts of models for safety assessment, efficacy testing, and mechanism of action studies, often necessitating genetically customized germ-free strains to mimic human disease conditions accurately.
Academic institutions and university research centers constitute a foundational customer base, driving the demand for basic research focused on understanding the fundamental interplay between the host genome, the environment, and the microbiota. These groups utilize germ-free mice to rigorously test specific scientific hypotheses, such as the direct causal link between a single bacterial species and an immunological outcome. While their volume requirements may be smaller than corporate clients, academic research dictates future therapeutic targets and often relies on specific, complex gnotobiotic setups, including models for specific disease induction or colonization with complex human fecal samples.
Contract Research Organizations (CROs) serve as critical intermediaries and rapidly growing consumers of germ-free models. CROs cater to both pharmaceutical and academic clients who lack the internal infrastructure or expertise to conduct gnotobiotic studies in-house. They require consistent, high-quality supply to execute sophisticated preclinical programs, including large-scale screening of small molecules, personalized FMT studies, and specialized immunological assessments. Their expansion signals the increasing necessity of gnotobiotic services within the broader drug development pipeline, making them a high-volume, professionalized segment of the customer base.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | $255.4 Million USD |
| Market Forecast in 2033 | $468.9 Million USD |
| Growth Rate | 8.9% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Charles River Laboratories, Taconic Biosciences, The Jackson Laboratory (JAX), Envigo (Inotiv), Genoway, Transposagen Biopharmaceuticals, Biocytogen, Shanghai Model Organisms Center, Janvier Labs, Crown Bioscience, Cyagen Biosciences, Ozgene, Horizon Discovery (PerkinElmer), Hera BioLabs, Wuxi AppTec |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Germ Free Mice Market Key Technology Landscape
The technology landscape underpinning the Germ Free Mice market is centered around maintaining absolute sterility and enabling high-precision genetic manipulation within this sterile environment. The foundation of this technology relies on advanced gnotobiotic housing systems, predominantly flexible film or rigid wall isolators, which employ positive pressure and high-efficiency particulate air (HEPA) filtration to prevent microbial ingress. These systems must be coupled with complex sterilization technologies, including autoclaves, gamma irradiation, and chemical sterilization protocols, for all materials entering the barrier. Continuous environmental monitoring, including pressure differentials and microbiological assays, is crucial and relies on sophisticated sensor technology and real-time data logging to ensure the germ-free status is never compromised.
Beyond basic maintenance, the market is increasingly defined by the technological ability to create and preserve complex, genetically defined models in an axenic state. CRISPR/Cas9 technology has fundamentally accelerated the production of genetically customized Germ Free Mice (e.g., knockout or humanized models), allowing researchers to study the interaction between specific gene function and the introduced microbiota with unprecedented speed. Furthermore, the reliance on cryopreservation techniques, essential for preserving valuable germ-free strains and facilitating international transfer without jeopardizing the colony’s status, represents a critical technological service offered by major suppliers. The development of specialized, fortified diets that meet the nutritional needs of mice without introducing contaminants is also a niche, yet vital, technological component.
An emerging technological trend involves integrating automation and high-throughput phenotyping within the isolator environment. Robotics and automated systems are being deployed to minimize human handling, a major contamination risk, and to facilitate continuous data collection on behavior, metabolism, and immune status without breaking the sterile barrier. Coupled with advanced molecular diagnostic tools, such as quantitative PCR and next-generation sequencing, used for frequent and ultra-sensitive testing for microbial contamination, these technologies ensure the reliability and reproducibility of gnotobiotic studies. This integration of high-tech tools positions the market for significant scaling, enabling complex studies that were previously logistically impossible.
Regional Highlights
- North America: This region holds the largest market share, driven by unparalleled R&D expenditure from pharmaceutical giants and highly funded academic research institutions in the US. The presence of global market leaders (e.g., Charles River Laboratories, The Jackson Laboratory) and extensive federal grants (NIH, DoD) supporting microbiome and immunology research ensure continuous high demand for specialized gnotobiotic services and models.
- Europe: Europe is a major hub for collaborative, basic research, particularly in countries like the UK, Germany, and France. Growth is steady, supported by EU-funded projects (Horizon Europe) focusing on personalized medicine and chronic disease pathogenesis. The market is characterized by stringent animal welfare regulations, which favor high-quality, standardized models and contribute to premium pricing structures.
- Asia Pacific (APAC): APAC is the fastest-growing market, primarily fueled by massive investments from the governments of China and Japan into domestic biotechnology capabilities and the establishment of state-of-the-art gnotobiotic facilities. Rapid expansion of Contract Research Organizations (CROs) in this region, coupled with a focus on infectious disease research and traditional medicine validation, drives exponential growth in demand for both standardized and custom germ-free models.
- Latin America (LATAM): The market in LATAM is emerging, characterized by localized research needs and increasing academic collaborations with North American and European institutions. Growth is often concentrated in specialized biomedical research institutes in countries such as Brazil and Mexico, focusing on regional infectious diseases and basic immunological studies.
- Middle East and Africa (MEA): This region represents the smallest market share but shows potential growth tied to rising investments in local healthcare and biomedical research infrastructure, particularly in the UAE and Saudi Arabia. Market activity is often driven by technology transfer and acquisition of expertise from global suppliers to build localized capacity for preclinical testing and disease modeling.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Germ Free Mice Market.- Charles River Laboratories
- Taconic Biosciences
- The Jackson Laboratory (JAX)
- Envigo (Inotiv)
- Janvier Labs
- Biocytogen
- Genoway
- Crown Bioscience
- Shanghai Model Organisms Center
- Cyagen Biosciences
- Ozgene
- Transposagen Biopharmaceuticals
- Hera BioLabs
- WuXi AppTec
- Horizon Discovery (PerkinElmer)
- GemPharmatech
- Ingenious Targeting Laboratory
- PolyGene
- Applied StemCell
- Lexicon Pharmaceuticals
Frequently Asked Questions
Analyze common user questions about the Germ Free Mice market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary difference between SPF and Germ Free Mice?
Specific Pathogen Free (SPF) mice are free only from a defined list of pathogens, retaining a complex, undefined microbiota. Germ Free (Axenic) mice are entirely free of all detectable microorganisms (bacteria, viruses, fungi, parasites) and are maintained in complete isolation, providing a sterile baseline necessary for colonization studies.
Which research applications drive the highest demand for Germ Free Mice?
The highest demand is currently driven by Gut Microbiome Research, followed closely by Immunology and Oncology. Researchers utilize these models to understand how specific microbial communities modulate immune responses, influence cancer progression, or affect drug metabolism and toxicity.
How significant is the role of CROs in the Germ Free Mice supply chain?
Contract Research Organizations (CROs) are increasingly critical, providing specialized gnotobiotic services and managing complex preclinical studies for pharmaceutical companies and academic labs that lack in-house containment facilities. They ensure access and standardization, acting as a major growth engine for the service segment.
What are the major technological challenges in maintaining germ-free status?
The main technological challenge involves preventing contamination, requiring continuous positive pressure maintenance in isolators, rigorous sterilization of all inputs (food, water, air), and employing advanced molecular diagnostics to detect ultra-low levels of contamination rapidly before colony loss occurs.
How does genetic modification (CRISPR/Cas9) integrate with Germ Free Mice production?
CRISPR/Cas9 technology enables the rapid generation of customized, genetically altered mice (knockouts, transgenics). These genetically complex models are subsequently derived and maintained in a germ-free state, allowing scientists to study the synergistic effects of specific genetic backgrounds and defined microbial exposure precisely.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
