Giardia Test Kit Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 431718 | Date : Dec, 2025 | Pages : 255 | Region : Global | Publisher : MRU
Giardia Test Kit Market Size
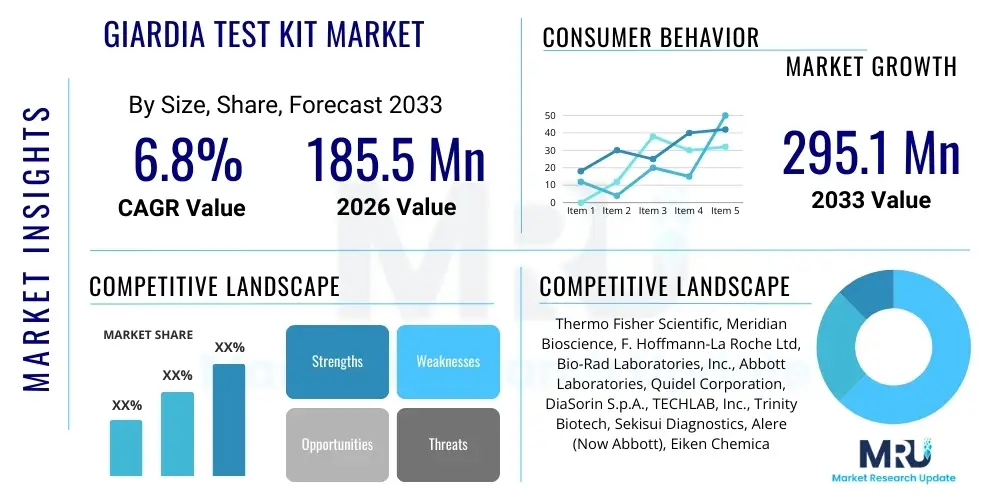
The Giardia Test Kit Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2026 and 2033. The market is estimated at USD 185.5 million in 2026 and is projected to reach USD 295.1 million by the end of the forecast period in 2033. This consistent expansion is underpinned by increasing global awareness regarding waterborne parasitic diseases and the imperative for rapid, accurate diagnosis in both human clinical settings and veterinary medicine. The market growth trajectory reflects a sustained shift from traditional diagnostic methods, such as microscopy, toward advanced, high-throughput immunoassay and molecular testing platforms, driving greater accessibility and improving public health outcomes across diverse geopolitical landscapes.
Giardia Test Kit Market introduction
The Giardia Test Kit Market encompasses specialized diagnostic tools utilized for the detection of Giardia duodenalis, a prevalent flagellated protozoan responsible for giardiasis, a significant gastrointestinal illness affecting humans and animals worldwide. These kits facilitate the identification of Giardia antigens or nucleic acids in fecal samples, minimizing diagnostic turnaround time compared to traditional methods. Key products include enzyme-linked immunosorbent assay (ELISA) kits, rapid immunochromatographic assays (RIAs), and Polymerase Chain Reaction (PCR) assays, each offering varying levels of sensitivity, specificity, and operational complexity suitable for different laboratory environments and testing requirements, ranging from point-of-care applications to high-volume reference laboratories.
Major applications of these diagnostic tools span human clinical diagnostics, where accurate identification is crucial for timely treatment and infection control, veterinary health (particularly in companion animals and livestock), and public health surveillance, including critical water quality testing to monitor contamination sources. The primary benefits of employing these advanced test kits include improved diagnostic precision, speed, and standardization across various testing facilities, which collectively enhance patient management and facilitate rapid intervention strategies to control outbreaks. The standardization offered by commercial kits reduces reliance on technician skill inherent in microscopic examination, a notable advantage in resource-constrained settings.
Driving factors propelling market expansion are multifaceted, including the escalating incidence of waterborne diseases globally, particularly in developing regions with inadequate sanitation infrastructure, and the growing integration of automated laboratory workflows demanding high-throughput diagnostic solutions. Furthermore, increasing regulatory focus on food and water safety, coupled with the heightened awareness among pet owners regarding zoonotic transmission risks, significantly contributes to the demand for reliable Giardia diagnostic tools. The continuous development of multiplex assays capable of simultaneously detecting multiple enteric pathogens alongside Giardia further enhances the utility and market penetration of these advanced testing solutions, streamlining the diagnostic process in clinical environments.
Giardia Test Kit Market Executive Summary
The Giardia Test Kit Market demonstrates robust growth driven primarily by technological advancements in rapid diagnostics and increased government investment in epidemiological surveillance programs globally. Business trends indicate a strong move toward decentralized testing models, favoring rapid immunochromatographic tests for field use and high-sensitivity molecular assays (PCR) for confirmation in centralized settings, leading major manufacturers to prioritize portfolio diversification across these technological segments. Strategic alliances between diagnostic developers and water utility companies are increasingly common, focusing on novel testing solutions optimized for environmental monitoring, marking a crucial expansion avenue beyond traditional healthcare sectors. Furthermore, mergers and acquisitions remain prevalent as companies seek to consolidate market share and acquire proprietary molecular diagnostic technologies to gain a competitive edge in high-value clinical segments.
Regionally, North America and Europe maintain leading positions, characterized by high healthcare expenditure, established diagnostic infrastructure, and stringent regulatory standards for clinical and veterinary diagnostics. However, the Asia Pacific (APAC) region is poised for the fastest growth, propelled by rapidly improving healthcare access, large patient populations susceptible to giardiasis due to environmental factors, and substantial governmental initiatives aimed at improving sanitation and infectious disease control. This regional shift mandates that market players establish robust distribution networks and adapt product offerings to suit the varied infrastructure and pricing sensitivities found across countries like India and China, necessitating a flexible market entry strategy focused on scalability and cost-effectiveness.
Segmentation trends highlight the dominance of Immunoassay Kits (ELISA and Rapid Tests) due to their cost-effectiveness and ease of use, particularly in non-reference laboratories and veterinary clinics. However, the Molecular Diagnostic segment (PCR) is exhibiting the highest growth rate, driven by demand for superior sensitivity and specificity, especially in cases requiring pathogen load quantification or species differentiation. End-user analysis reveals that Diagnostic Laboratories constitute the largest consumer base, utilizing these kits for large-scale processing, while the Veterinary Clinics segment is growing rapidly, reflecting rising disposable incomes allocated toward pet health and preventive care. Innovation is concentrated on developing multiplex panel tests that can screen for Giardia along with other common co-infecting parasites and bacteria, enhancing diagnostic efficiency and optimizing patient outcomes.
AI Impact Analysis on Giardia Test Kit Market
User inquiries regarding the role of Artificial Intelligence in the Giardia Test Kit Market frequently revolve around optimizing diagnostic workflow, improving data interpretation reliability, and accelerating outbreak response capabilities. Key concerns center on whether AI can truly enhance the precision of existing diagnostic tools, particularly automated ELISA readers and digital microscopy systems, and how machine learning algorithms can be applied to large-scale epidemiological data derived from diagnostic tests to predict outbreak patterns. Expectations are high that AI will transform quality control and standardization across decentralized testing sites, reducing human error and ensuring consistent diagnostic results globally. Users also seek information on AI-driven development platforms that could potentially accelerate the design and validation of next-generation multiplex test panels, thereby shortening the time-to-market for novel diagnostic solutions designed to detect Giardia and co-pathogens simultaneously.
The immediate impact of AI is most visible in enhancing the utility of digital imaging systems used in conjunction with traditional diagnostic methods. For instance, sophisticated AI algorithms are being trained to automatically analyze digitized fecal sample images, significantly improving the speed and accuracy of microscopic identification of Giardia cysts, compensating for the high variability associated with manual slide examination. Furthermore, in the realm of automated immunoassay platforms, AI systems monitor reagent stability, instrument performance, and subtle shifts in quality control metrics, ensuring the sustained reliability of high-volume testing environments. This integration minimizes false positive and false negative rates stemming from instrument drift or subtle procedural errors, thereby establishing a new benchmark for diagnostic confidence in large-scale clinical and environmental testing programs.
Looking ahead, AI’s greatest transformative potential lies in integrating diverse data streams—including diagnostic results from test kits, geographical data, climatic variables, and patient demographics—to establish predictive models for giardiasis transmission and seasonality. These models empower public health officials to deploy testing resources proactively to high-risk areas before significant outbreaks occur. Moreover, AI-powered software interfaces are being developed to simplify the interpretation of complex molecular diagnostic results, such as those from multiplex PCR assays, making sophisticated diagnostics more accessible to non-specialized laboratories. This technological evolution ensures that the intelligence derived from Giardia test kits is maximized, transitioning from a simple diagnostic result into actionable public health insights, thereby elevating the overall efficiency and responsiveness of disease surveillance systems.
- AI-driven optimization of automated immunoassay readers, ensuring superior consistency and reduced intra-assay variability.
- Machine Learning algorithms deployed for enhanced digital microscopy, automating cyst detection and quantification in fecal samples.
- Predictive modeling using diagnostic data to forecast giardiasis outbreaks, aiding proactive resource allocation and intervention strategies.
- Improved quality control and instrument calibration monitoring in high-throughput laboratories utilizing sophisticated analytical AI tools.
- Acceleration of novel test kit development through AI simulation and optimization of antigen/antibody binding characteristics.
DRO & Impact Forces Of Giardia Test Kit Market
The Giardia Test Kit Market is strongly influenced by a combination of powerful drivers centered on public health urgency and technological advancements, yet it faces persistent restraints primarily related to cost and market access challenges in developing economies. The primary driver is the globally increasing awareness and incidence of waterborne parasitic infections, necessitating reliable and prompt diagnostic solutions. Simultaneously, opportunities arise from the convergence of diagnostic technologies, notably the development of highly integrated, multiplexed molecular platforms capable of screening for numerous enteric pathogens, including Giardia, within a single test run. The overall impact force analysis suggests that while cost and infrastructure remain restraining factors, the overwhelming need for superior diagnostic accuracy and speed in mitigating zoonotic and public health risks will continue to propel market expansion robustly throughout the forecast period, especially in high-growth APAC and MEA markets.
Drivers include stringent regulatory requirements imposed by bodies such as the FDA and EU concerning food and water safety, compelling frequent environmental monitoring using certified Giardia tests. Furthermore, rising awareness among veterinary professionals and pet owners about the zoonotic potential of Giardia has significantly increased the demand for dedicated veterinary diagnostic kits. Technological innovation, specifically the commercialization of simpler, field-deployable rapid tests with improved sensitivity (approaching laboratory-grade PCR standards), enhances utility in diverse settings, from remote veterinary clinics to community water testing points. These factors collectively create a favorable environment for sustained investment and market adoption of advanced testing solutions, overcoming the historical reliance on low-sensitivity microscopic methods.
Restraints primarily revolve around the relatively high cost of advanced molecular diagnostic kits (e.g., real-time PCR) compared to conventional microscopy, posing a significant barrier to widespread adoption in low-income settings where giardiasis prevalence is often highest. Additionally, challenges related to sample collection, storage, and transport, particularly in geographically expansive or remote regions, can compromise sample integrity and test accuracy, thereby limiting the effectiveness of commercial test kits. Opportunities, however, exist in exploiting the growing trend towards personalized medicine and point-of-care diagnostics, where manufacturers can introduce highly portable, automated sample-to-result platforms that minimize handling steps and reduce the requirement for specialized laboratory infrastructure, effectively mitigating current logistical and cost restraints through design efficiency.
Segmentation Analysis
The Giardia Test Kit Market is comprehensively segmented based on product type, application, and end-user, providing a granular view of market dynamics and adoption patterns across different sectors. This structure reflects the varied technological maturity and operational requirements of diverse testing environments, from high-throughput centralized laboratories utilizing complex molecular techniques to veterinary practices demanding quick, reliable point-of-care results. Analyzing these segments reveals shifting preferences; while traditional immunoassays remain the volumetric leader due to widespread accessibility and lower cost, the molecular diagnostic segment is rapidly gaining market share driven by clinical requirements for higher sensitivity and specificity, particularly in managing chronic or atypical giardiasis cases where parasite load may be low or intermittent.
The segmentation by product type—encompassing Immunoassay Kits, Molecular Diagnostic Kits, and Other Tests—is vital for understanding the technological landscape and pricing sensitivities. Immunoassay Kits, including both ELISA and lateral flow devices (Rapid Tests), cater to both screening and quick diagnosis due to their relative simplicity and affordability. Conversely, Molecular Diagnostic Kits, predominantly PCR-based, are positioned as premium offerings utilized for confirmatory testing, epidemiological studies, and environmental surveillance where the lowest detection limits are required. Segmentation by application—Human Diagnostics, Veterinary Diagnostics, and Environmental Testing—highlights the diverse end-market drivers, with increasing zoonotic awareness boosting the veterinary segment, and water safety concerns driving the environmental sector’s demand for high-specificity tests capable of detecting cysts even in heavily filtered water samples.
Furthermore, segmenting by end-user—Diagnostic Laboratories, Hospitals, Veterinary Clinics, and Academic & Research Institutes—clarifies the key purchasing power centers and infrastructure requirements. Diagnostic Laboratories constitute the dominant segment, benefiting from scale and automation capabilities, while Veterinary Clinics represent a dynamic growth area, driven by the increasing professionalization of pet care. Understanding these distinct segment needs allows manufacturers to tailor marketing strategies, optimize product features (e.g., portability vs. automation), and manage pricing strategies effectively to maximize market penetration across the highly diverse global landscape of giardiasis testing, ensuring products align optimally with the operational constraints and diagnostic goals of each end-user category.
- By Product Type:
- Immunoassay Kits (ELISA, Rapid Immunochromatographic Tests)
- Molecular Diagnostic Kits (PCR/qPCR, DNA Microarrays)
- Other Tests (Microscopy Ancillary Reagents, Culture Kits)
- By Application:
- Human Diagnostics
- Veterinary Diagnostics (Companion Animals, Livestock)
- Environmental Testing (Water Quality, Food Safety)
- By End-User:
- Diagnostic Laboratories (Reference & Clinical Labs)
- Hospitals and Clinics
- Veterinary Clinics and Hospitals
- Academic and Research Institutes
Value Chain Analysis For Giardia Test Kit Market
The Value Chain for the Giardia Test Kit Market starts with upstream activities involving the sourcing and manufacturing of critical biological and chemical reagents, including specialized antibodies, purified antigens, and high-quality molecular components such as primers and probes. This stage is crucial as the quality and stability of these core components directly dictate the sensitivity and reliability of the final test kit product. Key players in this phase focus heavily on proprietary antigen production and stringent quality control protocols to ensure consistency across manufacturing batches, often relying on specialized biotechnology suppliers. The subsequent midstream phase involves the complex assembly and packaging of the test kits, including optimizing the physical format (e.g., lateral flow membrane assembly, microplate coating) and obtaining necessary regulatory approvals (e.g., CE Mark, FDA clearance), which represents a significant value addition step requiring specialized manufacturing facilities and intellectual property protection.
Downstream activities center on distribution, sales, and post-sale support, representing the interface with the end-users. The distribution channel structure is bifurcated into direct sales channels, typically used for large-volume institutional customers such as government laboratories and major hospital systems, and indirect channels relying on specialized regional medical distributors and veterinary suppliers. Indirect channels are crucial for penetrating fragmented markets and smaller clinics, leveraging the distributor's established logistical networks and local market expertise. The final layer of the value chain involves effective market outreach, technical training for end-users on protocol execution, and responsive customer support to manage inquiries related to test performance and troubleshooting, which contributes significantly to brand loyalty and repeat purchases, especially in the context of sensitive diagnostic products.
Direct distribution offers manufacturers greater control over pricing and customer relationships, which is highly advantageous for premium molecular diagnostics where complex technical support is required. However, the expansive geographic reach required for global market coverage necessitates a strong reliance on indirect distribution partners, particularly in emerging economies where local market access and understanding of regulatory nuances are critical. Successful market players strategically manage this balance, investing heavily in digital platforms and logistics to ensure cold-chain integrity for sensitive reagents while simultaneously training their indirect sales partners extensively on product differentiation and standardized testing procedures. This integrated approach ensures the efficiency of the supply chain from raw material sourcing to end-user application, maximizing the test kit's utility and market impact.
Giardia Test Kit Market Potential Customers
The primary customers for Giardia Test Kits span institutional healthcare, specialized veterinary medicine, and public health infrastructure, each driven by distinct testing needs and volume requirements. Clinical diagnostic laboratories, encompassing both centralized reference laboratories and smaller hospital labs, represent the largest volume consumers. These entities require high-throughput, automated solutions, such as ELISA and automated PCR platforms, to handle large volumes of human fecal samples, emphasizing speed, cost per test, and integration with Laboratory Information Management Systems (LIMS). Their purchasing decisions are heavily influenced by regulatory compliance, accreditation standards, and demonstrated clinical performance metrics like high sensitivity and specificity in diverse patient populations, making validation data a critical sales factor.
The veterinary sector constitutes a rapidly expanding customer base, particularly small animal veterinary clinics and large corporate veterinary hospital chains. Driven by increased companion animal ownership and heightened awareness of zoonotic diseases, these customers primarily favor rapid immunochromatographic assays (lateral flow tests) for quick, on-the-spot screening of symptomatic pets. They prioritize ease of use, short turnaround time (TAT), and minimal requirement for specialized equipment, allowing for immediate diagnosis and treatment planning during routine office visits. Furthermore, large livestock farms and associated diagnostic services utilize these kits for monitoring herd health and preventing widespread parasitic infections that can severely impact agricultural productivity and food safety, requiring solutions optimized for high sample batch processing.
Public health agencies, including municipal water treatment facilities, environmental monitoring organizations, and academic research institutions, form the third major segment. Water quality testing demands the highest sensitivity, often necessitating advanced molecular diagnostics (qPCR) capable of detecting low concentrations of Giardia cysts in complex environmental samples like treated wastewater or drinking water reservoirs. These customers often procure kits through government tenders and grant funding, emphasizing reliability, official certifications, and the capability of the tests to adhere strictly to international water safety guidelines (e.g., EPA or WHO standards). Academic and research institutes utilize kits for epidemiological studies, vaccine development, and testing novel drug efficacy, focusing on maximum analytical precision and specialized molecular capabilities for strain typing and genomic analysis.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 185.5 Million |
| Market Forecast in 2033 | USD 295.1 Million |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Thermo Fisher Scientific, Meridian Bioscience, F. Hoffmann-La Roche Ltd, Bio-Rad Laboratories, Inc., Abbott Laboratories, Quidel Corporation, DiaSorin S.p.A., TECHLAB, Inc., Trinity Biotech, Sekisui Diagnostics, Alere (Now Abbott), Eiken Chemical Co., Ltd., GeneProof a.s., BioFire Diagnostics (A bioMérieux Company), Savyon Diagnostics, Hardy Diagnostics, VedaLab, Trivitron Healthcare, Creative Diagnostics, BioCheck Inc. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Giardia Test Kit Market Key Technology Landscape
The technological landscape of the Giardia Test Kit Market is dominated by three principal platforms: immunoassays, molecular diagnostics (PCR), and emerging rapid diagnostic technologies. Immunoassays, particularly Enzyme-Linked Immunosorbent Assay (ELISA) and rapid lateral flow assays, utilize specific antibodies to detect Giardia-specific antigens (e.g., Giardia Cysteine-Rich Proteins - GCRP) in stool samples. ELISA offers high sensitivity and is scalable for batch testing in clinical laboratories, whereas rapid tests provide results within minutes at the point-of-care, sacrificing some sensitivity for speed and portability. Ongoing innovation in this sector focuses on improving the stability of conjugated antibodies and incorporating advanced nanomaterials to enhance the visibility and quantification of the test line, thereby bridging the performance gap between traditional ELISA and rapid lateral flow platforms without increasing complexity.
Molecular diagnostics, primarily real-time Polymerase Chain Reaction (qPCR) and nested PCR, represent the gold standard for sensitivity and specificity due to their ability to directly detect and amplify Giardia DNA or RNA sequences. This technology is critical for environmental testing, where parasite concentrations are extremely low, and for clinical diagnosis in complex cases where intermittent shedding of cysts might lead to false negatives with less sensitive methods. The current technological evolution is moving toward multiplex PCR systems, such as those integrated into closed cartridge systems (e.g., BioFire FilmArray), which allow for the simultaneous detection of Giardia and dozens of other enteric pathogens from a single sample. These automated systems minimize hands-on time, reduce the risk of cross-contamination, and provide comprehensive results suitable for critically ill patients.
An increasingly important segment involves next-generation sequencing (NGS) and advanced microfluidic technologies, although these are currently confined mostly to high-end reference laboratories and research settings. NGS is used for definitive genotyping of Giardia isolates, which is invaluable for epidemiological tracking and understanding transmission dynamics between hosts (genotyping A, B, C, D, etc.). Microfluidics are instrumental in developing true sample-to-answer testing devices, integrating sample preparation, amplification, and detection onto a single, disposable chip. This focus on miniaturization, automation, and consolidation of multiple testing steps signifies the future trajectory of the market, promising highly accurate diagnostics deployable in low-resource settings, thus expanding the geographic accessibility of premium Giardia testing capabilities.
Regional Highlights
The global Giardia Test Kit Market exhibits significant regional variation in adoption, driven by healthcare infrastructure maturity, disease prevalence, regulatory frameworks, and public health spending. North America, specifically the United States and Canada, holds the largest market share, predominantly due to high consumer spending on advanced healthcare, the strong presence of major diagnostic companies, and rigorous regulatory requirements for food and water safety. The region shows high adoption of automated molecular diagnostics in reference laboratories and widespread use of professional-grade rapid tests in the expansive veterinary market, focusing heavily on continuous technological upgrades and quality assurance, maintaining a preference for FDA-cleared and high-throughput solutions.
Europe represents the second-largest market, characterized by advanced public healthcare systems and strong public health surveillance networks, particularly in Western European nations like Germany, France, and the UK. The market here is sustained by structured epidemiological monitoring programs and widespread use of CE-IVD marked immunoassay and PCR kits. There is a notable emphasis on standardization across member states, driving demand for harmonized, validated diagnostic protocols. Conversely, the Asia Pacific (APAC) region is forecasted to experience the highest growth rate, fueled by rapid urbanization, sanitation challenges leading to high endemic giardiasis prevalence, and increasing governmental investments aimed at expanding infectious disease diagnostic capabilities in countries like China, India, and Southeast Asia, creating immense potential for cost-effective rapid diagnostic kits and scalable ELISA platforms.
Latin America, the Middle East, and Africa (MEA) present unique market dynamics. Latin American countries are seeing increased market penetration, often relying on imported kits, driven by localized giardiasis outbreaks linked to contaminated water sources. The MEA region, particularly parts of Africa, represents a high-need market due to widespread water safety issues, but adoption is often constrained by funding limitations, infrastructure deficits, and logistical challenges. In these markets, there is a strong demand for low-cost, robust, shelf-stable rapid diagnostic tests that require minimal technical expertise and no reliance on cold-chain logistics, necessitating different product design considerations focused on maximizing durability and affordability to achieve widespread public health impact and accessibility.
- North America: Dominant market share due to mature healthcare infrastructure, high molecular test adoption, and strong veterinary diagnostic sector demand.
- Europe: High adoption of centralized testing, stringent regulatory standards (CE-IVD), and significant governmental focus on preventative public health measures and surveillance.
- Asia Pacific (APAC): Highest growth trajectory, driven by high disease prevalence, improving healthcare access, and large-scale public health programs in countries like India and China demanding scalable, affordable solutions.
- Latin America: Growing demand influenced by local endemicity and improving diagnostic capabilities, requiring robust, reliable kits capable of addressing infrastructure variability.
- Middle East and Africa (MEA): Emerging market characterized by high public health needs; demand focused on extremely cost-effective, durable rapid tests suitable for resource-limited, decentralized testing environments.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Giardia Test Kit Market.- Thermo Fisher Scientific
- Meridian Bioscience
- F. Hoffmann-La Roche Ltd
- Bio-Rad Laboratories, Inc.
- Abbott Laboratories
- Quidel Corporation
- DiaSorin S.p.A.
- TECHLAB, Inc.
- Trinity Biotech
- Sekisui Diagnostics
- Alere (Now Abbott)
- Eiken Chemical Co., Ltd.
- GeneProof a.s.
- BioFire Diagnostics (A bioMérieux Company)
- Savyon Diagnostics
- Hardy Diagnostics
- VedaLab
- Trivitron Healthcare
- Creative Diagnostics
- BioCheck Inc.
Frequently Asked Questions
Analyze common user questions about the Giardia Test Kit market and generate a concise list of summarized FAQs reflecting key topics and concerns.What are the primary technological differences between Immunoassay and PCR Giardia Test Kits?
Immunoassay Kits (ELISA, Rapid Tests) detect specific Giardia antigens (proteins) in the sample, offering quick results and lower costs suitable for high-volume screening. Conversely, PCR (Molecular Diagnostic Kits) detects the parasite's genetic material (DNA), providing significantly higher sensitivity and specificity, making it the preferred method for confirmation, low-parasite load infections, and environmental water testing.
Which end-user segment drives the highest demand volume in the Giardia Test Kit Market?
Clinical and Reference Diagnostic Laboratories constitute the segment driving the highest overall volume demand. These laboratories leverage automated systems for high-throughput processing of both human and sometimes veterinary samples, primarily utilizing scalable platforms such as ELISA and automated PCR systems to meet large-scale testing requirements efficiently and cost-effectively.
How is Artificial Intelligence (AI) expected to influence the accuracy of Giardia diagnostics?
AI is expected to enhance diagnostic accuracy by optimizing the quality control processes in automated systems and improving the reliability of digital interpretation. AI algorithms can automate the analysis of digital microscopy images to identify Giardia cysts, reducing reliance on manual technician skill, and enabling predictive maintenance for complex laboratory instruments, thus minimizing error rates.
What is the main restraining factor limiting the adoption of advanced Giardia Test Kits in emerging markets?
The primary restraining factor is the high capital cost associated with purchasing advanced molecular diagnostic equipment (e.g., real-time PCR cyclers) and the ongoing cost per test. This financial barrier, coupled with inadequate laboratory infrastructure and inconsistent cold-chain logistics, limits the widespread adoption of high-sensitivity kits in resource-constrained developing economies where giardiasis prevalence is often severe.
Why is the Asia Pacific (APAC) region forecasted to exhibit the highest growth rate in this market?
APAC is projected to see the highest growth due to a combination of high endemic disease burden resulting from rapid urbanization and sanitation challenges, coupled with increasing governmental and private sector investment in expanding diagnostic healthcare infrastructure. This confluence creates a substantial and rapidly accessible market for both affordable rapid tests and higher-end molecular solutions aimed at public health surveillance and clinical management.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
