Hollow Microneedle Technology Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 433578 | Date : Dec, 2025 | Pages : 246 | Region : Global | Publisher : MRU
Hollow Microneedle Technology Market Size
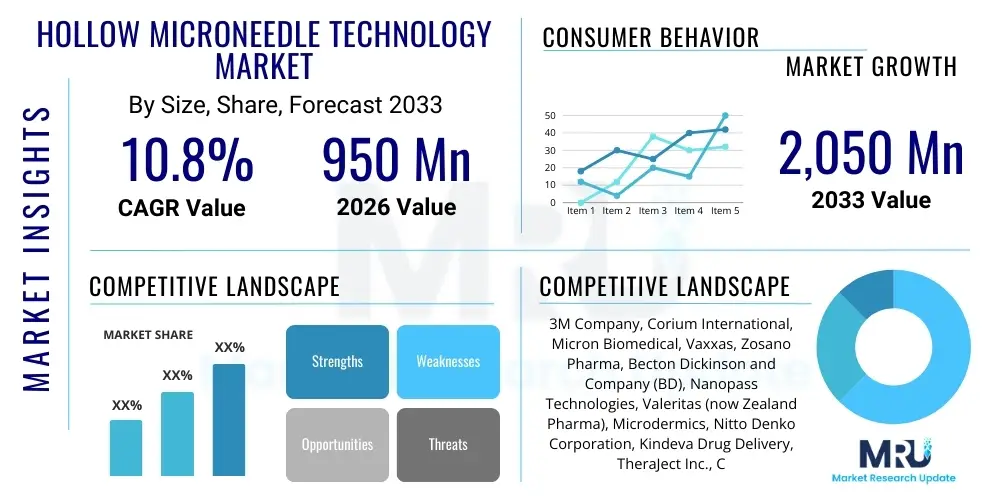
The Hollow Microneedle Technology Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 10.8% between 2026 and 2033. The market is estimated at USD 950 million in 2026 and is projected to reach USD 2,050 million by the end of the forecast period in 2033.
Hollow Microneedle Technology Market introduction
The Hollow Microneedle (HMN) Technology Market encompasses advanced transdermal drug delivery systems designed to penetrate the stratum corneum, the outermost layer of the skin, without reaching the underlying nerve endings, thus providing painless administration. Unlike solid microneedles used primarily for skin pretreatment or dissolving microneedles for localized drug release, HMNs function similarly to miniature hypodermic needles, allowing for precise and controlled delivery of liquid formulations, including biologics, vaccines, and insulin. This technology addresses major limitations associated with conventional needle injections, such as needle-stick injuries, patient non-compliance due to phobia, and the degradation of sensitive therapeutics within the gastrointestinal tract when administered orally. The primary advantages revolve around enhanced bioavailability, sustained release capabilities, and the potential for self-administration, positioning HMNs as a transformative tool in personalized medicine and vaccination efforts.
Major applications of HMN technology span several therapeutic areas, most notably in chronic disease management and immunization programs. In diabetes care, HMN patches are being developed for continuous or responsive insulin delivery, potentially revolutionizing patient compliance and glucose control. Furthermore, the capacity of HMNs to efficiently deliver vaccines—particularly subunit vaccines and those requiring cold chain maintenance—is a significant driving factor, offering opportunities for expanded global immunization coverage and simplified logistics. The aesthetic and dermatological fields also leverage HMNs for delivering complex cosmeceuticals and localized treatments for conditions like alopecia and hyperpigmentation, capitalizing on the high local concentration achievable through minimally invasive penetration.
The core driving factors propelling market growth include the rising prevalence of chronic diseases requiring frequent parenteral drug administration, such as diabetes and autoimmune disorders, coupled with increasing demand for patient-centric and minimally invasive drug delivery solutions. Technological advancements in microfabrication techniques, including lithography, etching, and molding (e.g., polymer micro-molding), have significantly reduced production costs and enhanced the scalability and reproducibility of high-precision microneedle arrays. Additionally, strategic collaborations between pharmaceutical giants and specialized microneedle device developers are accelerating clinical validation and commercialization, further embedding HMN technology into standard medical practice as an effective alternative to traditional injections.
Hollow Microneedle Technology Market Executive Summary
The Hollow Microneedle Technology Market is poised for substantial expansion driven by a strong shift toward self-administration therapeutics and innovative vaccine delivery methods. Key business trends indicate intensified investment in automated manufacturing processes to meet scalability requirements, particularly for high-volume applications like influenza and COVID-19 vaccines. Furthermore, strategic licensing agreements and M&A activities are focusing on integrating specialized HMN intellectual property into the pipelines of large pharmaceutical and biotechnology companies, mitigating development risks and accelerating time-to-market. The competitive landscape is characterized by innovation in material science, focusing on biocompatible polymers and enhanced tip stability, alongside the development of "smart" HMN systems capable of responsive or pulsatile drug release, moving beyond simple passive delivery systems.
Geographically, North America remains the dominant revenue generator, fueled by robust R&D infrastructure, high healthcare expenditure, and the early adoption of advanced medical devices, particularly in biotech centers focusing on novel large-molecule therapeutics. However, the Asia Pacific (APAC) region is projected to register the highest growth rate due to expanding healthcare access, governmental focus on mass immunization campaigns, and the establishment of manufacturing hubs in countries like China and India, making HMN technology an attractive low-cost, high-compliance delivery option. Europe follows, with growth underpinned by favorable regulatory pathways (e.g., EMA authorization) supporting advanced medical technologies and strong academic research institutions contributing to foundational breakthroughs.
Segment trends reveal that the Drug Delivery segment, specifically involving biologics and personalized medicines, holds the largest market share due to the high efficacy HMNs offer in maintaining the stability and integrity of sensitive drugs during transit across the skin barrier. Within applications, vaccine delivery is the fastest-growing subsegment, spurred by global health emergencies and the inherent advantages of microneedles in improving patient acceptability and reducing the logistical burden associated with traditional cold-chain injections. In terms of material, silicon-based microneedles, favored for their precision and strength, maintain a significant presence, though polymer-based systems are rapidly gaining traction due to their cost-effectiveness and enhanced biocompatibility profiles, catering especially to single-use patch applications.
AI Impact Analysis on Hollow Microneedle Technology Market
User inquiries regarding the role of Artificial Intelligence (AI) in Hollow Microneedle Technology frequently center on optimization, predictive modeling, and quality control. Users are keen to understand how AI algorithms can enhance the efficiency of HMN manufacturing processes, particularly in reducing defects during complex microfabrication and improving batch consistency. Another major theme is the use of AI in optimizing drug loading and release kinetics, asking if machine learning can predict the ideal needle geometry, material composition, or drug concentration for achieving specific pharmacological outcomes in varied patient populations. Furthermore, there is significant interest in how AI diagnostics can integrate with HMN monitoring systems to ensure proper patch application and compliance, thereby enhancing the overall efficacy and safety profile of these devices in clinical settings.
The integration of AI, machine learning (ML), and deep learning models is expected to revolutionize several critical stages of the HMN life cycle, from early design through post-market surveillance. In R&D, AI facilitates rapid screening of material candidates and predictive modeling of skin-device interaction, drastically reducing the experimental cycles traditionally required to finalize a successful HMN array design. By analyzing vast datasets related to skin characteristics, drug stability, and delivery rates, AI can customize needle specifications (length, density, tip geometry) to optimize penetration efficiency across different demographics, addressing a major challenge in transdermal delivery variability.
In manufacturing, AI-driven computer vision systems are being deployed for high-throughput, non-destructive quality inspection, detecting microscopic defects in needle tips and ensuring uniformity across production batches, which is crucial for maintaining clinical performance standards. Moreover, AI predictive maintenance models optimize equipment utilization and minimize downtime in specialized cleanroom environments. This technological synergy ensures that the transition from small-scale laboratory prototypes to mass-produced, commercially viable HMN patches is robust, cost-effective, and maintains the stringent quality required for pharmaceutical products, thereby accelerating market adoption and lowering long-term operational costs for manufacturers.
- AI-driven optimization of microfabrication parameters (etching depth, mold temperature) to minimize production defects and enhance array consistency.
- Machine learning models predicting optimal drug encapsulation efficiency and release profiles based on needle geometry and polymer type.
- Integration of AI algorithms with in-vivo monitoring systems to verify successful skin penetration and track adherence data in real-time.
- Predictive analytics for clinical trial design, identifying patient subgroups most likely to benefit from HMN administration methods.
- Automated visual inspection using deep learning for high-speed quality control of needle tip uniformity and structural integrity.
DRO & Impact Forces Of Hollow Microneedle Technology Market
The Hollow Microneedle Technology Market is shaped by a complex interplay of Drivers, Restraints, and Opportunities (DRO), collectively forming the Impact Forces that dictate market velocity. Key drivers include the overwhelming global demand for painless drug administration, particularly for pediatric and needle-phobic patient populations, coupled with the inherent logistical benefits HMNs offer for vaccine distribution in resource-limited settings by potentially eliminating the need for highly skilled administrators or stringent cold chain storage in some formulations. These market forces are amplified by significant investments from governmental and philanthropic organizations supporting the development of next-generation vaccine delivery technologies, ensuring a continuous flow of funding into R&D and clinical validation.
Despite these powerful drivers, several significant restraints currently impede faster market adoption. Primary among these is the regulatory uncertainty surrounding novel transdermal devices, as HMNs often straddle the categories of drug, device, and combination product, leading to prolonged and complex approval processes with agencies like the FDA and EMA. Furthermore, the high capital expenditure required for establishing specialized microfabrication facilities, which must adhere to stringent GMP standards, poses a barrier to entry for smaller innovative firms. Technical restraints also persist, including challenges related to maintaining the mechanical strength of the hollow needles during insertion and ensuring precise control over the administered drug volume, which can be difficult to manage compared to conventional syringe systems.
However, the market is rich with transformative opportunities. The most significant opportunity lies in the burgeoning field of personalized medicine and continuous drug delivery systems, particularly in endocrinology and oncology, where HMNs can be integrated with biosensors for closed-loop delivery. Additionally, expanding the application scope beyond traditional pharmaceuticals to include gene therapies, oligonucleotides, and cosmetic procedures presents high-growth avenues. The continuous refinement of materials, moving towards dissolvable or biodegradable hollow structures that eliminate biohazard waste, also provides a compelling trajectory for innovation, ultimately positioning HMN technology as a central component in future decentralized and patient-managed healthcare models.
Segmentation Analysis
The Hollow Microneedle Technology Market is highly segmented based on critical factors such as material, application, and end-user, reflecting the diverse utility and customization inherent in this drug delivery platform. Analyzing these segments provides strategic insights into areas of highest growth potential and technological focus. The segmentation based on material (Silicon, Polymer, Metal) dictates the manufacturing process, cost structure, and mechanical properties, with polymers rapidly gaining preference for their biocompatibility and disposability. Application segmentation (Vaccine Delivery, Drug Delivery, Cosmetics) highlights the varying requirements for dose size and penetration depth. Finally, end-user segmentation (Hospitals, Clinics, Home Care Settings) underscores the shift toward decentralized healthcare and the growing significance of self-administration devices in managing chronic conditions.
- By Material:
- Silicon (Preferred for precision and mechanical strength)
- Polymer (Used for flexibility, biocompatibility, and low cost)
- Metal (Offers durability and stiffness)
- By Application:
- Vaccine Delivery (Influenza, COVID-19, HPV, Polio)
- Drug Delivery (Insulin, Hormones, Biologics, Pain Management)
- Cosmetics and Aesthetics (Anti-aging serums, localized treatments)
- Diagnostics and Monitoring (Interstitial fluid sampling)
- By End-User:
- Hospitals and Clinics
- Academic and Research Institutes
- Home Care Settings and Self-Administration
- By Manufacturing Technique:
- Molding (Polymer processing)
- Lithography and Etching (Silicon and Metal processing)
- Drawn Techniques
Value Chain Analysis For Hollow Microneedle Technology Market
The value chain for Hollow Microneedle Technology is complex and highly specialized, beginning with the upstream supply of ultra-high-purity raw materials and extending through sophisticated microfabrication to patient-centric distribution. Upstream activities are dominated by providers of specialized materials, including medical-grade polymers (e.g., PLGA, PLA), silicon wafers, and specific metal alloys suitable for micro-manufacturing processes. Reliability and consistency in raw material supply are critical, as any variation can drastically impact the precision and mechanical integrity of the finished needles. This stage also includes the development and supply of high-precision micro-molding and etching equipment, which often requires significant customization and proprietary technology from specialized engineering firms.
The midstream segment involves the core intellectual property and manufacturing expertise, where device developers transform raw materials into sterile, functional HMN arrays, followed by the integration of the drug formulation (loading) into the device reservoir. This phase is characterized by stringent quality control requirements and relies heavily on automated cleanroom manufacturing (ISO standards). Contract Development and Manufacturing Organizations (CDMOs) specializing in micro-scale production play a vital role here, often partnering with pharmaceutical companies (the downstream customers) to scale up production under Good Manufacturing Practices (GMP). The ability to efficiently scale the manufacturing process while maintaining nanometer-level precision is the primary value driver at this stage.
Downstream analysis focuses on distribution and final market penetration. Distribution channels are predominantly indirect, leveraging established pharmaceutical wholesalers and specialized medical device distributors to reach hospitals, clinics, and pharmacies globally. However, for devices intended for self-administration in home care settings—a rapidly growing segment—direct-to-patient channels, including specialized online pharmacies or mail-order services, are gaining importance. The successful commercialization hinges on effective clinical support, physician training, and patient education regarding proper application techniques, ensuring high compliance and optimal therapeutic outcomes, which ultimately drives prescription rates and market uptake. Direct salesforces are crucial for targeting large hospital systems and key opinion leaders (KOLs) in specific therapeutic areas.
Hollow Microneedle Technology Market Potential Customers
The primary potential customers for Hollow Microneedle Technology are multi-faceted, ranging from large pharmaceutical corporations seeking innovative delivery methods for their proprietary therapeutics to governmental agencies focused on public health initiatives. Pharmaceutical companies represent the largest buying power, primarily driven by the need to extend patent life, improve patient adherence, and deliver complex large-molecule drugs (e.g., monoclonal antibodies, peptide hormones) that cannot be absorbed orally. These corporations acquire HMN technology either through in-house development, licensing agreements, or outsourcing manufacturing to specialized HMN device firms, viewing the technology as a competitive differentiator in crowded therapeutic markets.
Another significant customer segment includes academic research institutions and biotechnology startups focused on drug discovery and early-stage clinical development. These entities procure HMN prototypes and small-scale manufacturing services to validate novel drug candidates and explore localized delivery strategies in pre-clinical and Phase I trials. Their purchasing decisions are often based on technical capability, material versatility, and the ability of the HMN platform to handle diverse chemical and biological payloads, driving demand for customizable and highly precise devices.
End-users, such as hospitals, dermatology clinics, and increasingly, individual consumers utilizing home care devices, constitute the final point of purchase. Hospitals and clinics purchase HMN systems for efficient, less painful immunization programs and managing chronic diseases like diabetes (insulin patches). The fastest emerging customer base, however, is the individual patient managing chronic conditions at home, where ease of use, reduced pain, and the ability for self-administration are paramount purchasing criteria, positioning the HMN market strongly within the decentralized healthcare movement.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 950 Million |
| Market Forecast in 2033 | USD 2,050 Million |
| Growth Rate | 10.8% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | 3M Company, Corium International, Micron Biomedical, Vaxxas, Zosano Pharma, Becton Dickinson and Company (BD), Nanopass Technologies, Valeritas (now Zealand Pharma), Microdermics, Nitto Denko Corporation, Kindeva Drug Delivery, TheraJect Inc., Clearside Biomedical, Takeda Pharmaceutical Company (Selected Assets), CosMED Pharmaceutical Co., Ltd., LTS Lohmann Therapie-Systeme AG, Small Science Labs, MyLife Technologies B.V. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Hollow Microneedle Technology Market Key Technology Landscape
The technological landscape of the Hollow Microneedle market is characterized by sophisticated micro-engineering techniques derived largely from the semiconductor industry, coupled with innovations in material science and drug formulation. Fabrication techniques are crucial, with Deep Reactive Ion Etching (DRIE) dominating the production of high-aspect-ratio silicon microneedles, offering unparalleled precision and mechanical strength for robust skin penetration. However, for mass production of disposable, low-cost patches, precision micro-molding, utilizing UV lithography combined with specialized polymer casting, is increasingly preferred. This polymer approach facilitates the integration of complex features, such as controlled flow channels and drug reservoirs, directly into the microneedle structure, optimizing the fluid dynamics for efficient drug infusion.
Advancements are also centered on optimizing the fluid flow and drug-loading mechanisms specific to hollow structures. Research efforts are focused on improving the anti-clogging design of the hollow bore, particularly when delivering high-viscosity or particulate drug suspensions. This involves engineering the internal geometry of the lumen and potentially incorporating specialized coatings to ensure uniform and complete dose delivery. Furthermore, the development of integrated drug reservoirs and actuators—ranging from simple pressure-sensitive adhesives to complex micro-pumps—is crucial for enabling controlled, sustained, or on-demand delivery profiles, moving HMN patches closer to sophisticated wearable drug delivery systems.
Crucially, the next generation of HMN technology emphasizes enhanced usability and connectivity. This includes the incorporation of sensors or indicators that confirm successful needle insertion and dosage completion, addressing concerns related to user error in self-administration. Connectivity, achieved through Bluetooth or near-field communication (NFC) capabilities, allows for tracking compliance, monitoring real-time delivery performance, and integrating data into electronic health records (EHRs). This convergence of micro-device engineering, digital health, and specialized material science defines the cutting edge of the HMN market, driving both efficacy improvements and broader patient acceptance.
Regional Highlights
Geographic analysis reveals pronounced differences in adoption rates, regulatory environments, and market maturity across global regions, significantly influencing the overall growth trajectory of the Hollow Microneedle Technology Market. North America, specifically the United States, holds the dominant market share, primarily driven by a robust and highly funded biotechnology and pharmaceutical sector that actively seeks novel drug delivery solutions for high-value biologics and specialty drugs. The presence of major device manufacturers and a clear trend toward outsourcing complex pharmaceutical manufacturing and delivery innovation within this region sustains its leadership. Furthermore, the region benefits from strong governmental support for biomedical research and a healthcare system capable of absorbing the higher initial costs associated with premium, cutting-edge drug delivery technologies, coupled with a high prevalence of chronic diseases requiring advanced therapeutic management.
The European market represents a significant growth area, characterized by a stringent but supportive regulatory environment through the European Medicines Agency (EMA), which facilitates the adoption of scientifically validated medical devices. Western European countries, particularly Germany, the UK, and France, exhibit strong capabilities in pharmaceutical R&D and precision engineering, fostering collaboration between academia and industry on HMN development. The growth in Europe is also fueled by public healthcare systems seeking cost-effective, high-compliance alternatives to traditional injections, particularly in large-scale vaccination programs, driving demand for scalable polymer-based HMN systems.
Asia Pacific (APAC) is projected to emerge as the fastest-growing region during the forecast period. This accelerated growth is attributed to massive, untapped patient pools, increasing healthcare infrastructure spending, and a strategic focus by governments in countries like Japan, South Korea, and China on developing high-technology medical manufacturing capabilities. The region's inherent challenges in drug distribution and patient compliance in vast, rural areas make the stability and ease-of-use offered by HMN patches exceptionally valuable. Furthermore, the strong presence of generic and biosimilar manufacturers is driving the search for differentiated delivery platforms, positioning APAC as a crucial hub for low-cost, high-volume production and utilization of Hollow Microneedle Technology.
- North America: Dominates the market due to high R&D spending, established presence of pharmaceutical innovators, and high disposable income facilitating adoption of premium delivery systems.
- Europe: Characterized by strong academic research and supportive regulatory frameworks, driving adoption in vaccine delivery and clinical trials for chronic diseases.
- Asia Pacific (APAC): Fastest growing market segment, propelled by massive population immunization needs, expanding healthcare access, and regional focus on scaling advanced manufacturing techniques.
- Latin America (LATAM): Emerging market driven by improving healthcare access and government initiatives focused on minimizing needle-stick injuries and enhancing patient safety in hospital settings.
- Middle East & Africa (MEA): Growth focused on addressing critical public health needs, particularly in vaccine delivery and insulin management, where the simplified administration offers logistical advantages.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Hollow Microneedle Technology Market.- 3M Company
- Corium International
- Micron Biomedical
- Vaxxas
- Zosano Pharma
- Becton Dickinson and Company (BD)
- Nanopass Technologies
- Zealand Pharma (formerly Valeritas)
- Microdermics Inc.
- Nitto Denko Corporation
- Kindeva Drug Delivery
- TheraJect Inc.
- Clearside Biomedical
- Takeda Pharmaceutical Company (focusing on advanced delivery)
- CosMED Pharmaceutical Co., Ltd.
- LTS Lohmann Therapie-Systeme AG
- Small Science Labs
- MyLife Technologies B.V.
- PharmaTher Inc.
- Debiotech SA
Frequently Asked Questions
Analyze common user questions about the Hollow Microneedle Technology market and generate a concise list of summarized FAQs reflecting key topics and concerns.What are the primary advantages of Hollow Microneedle (HMN) technology over traditional injections?
Hollow Microneedle technology offers several key advantages, most notably painless drug delivery by penetrating only the stratum corneum without reaching nerve endings. This significantly improves patient compliance, especially among those with needle phobia. Additionally, HMNs reduce the risk of accidental needle-stick injuries and often allow for simpler self-administration of therapeutics, potentially stabilizing sensitive biologics and vaccines at room temperature for easier distribution.
How does the regulatory pathway for Hollow Microneedle devices typically differ from standard pharmaceuticals?
The regulatory pathway for Hollow Microneedles is often complex because they are usually classified as combination products (integrating a drug/biologic and a medical device). This necessitates dual submission requirements to regulatory bodies like the FDA and EMA, requiring comprehensive data demonstrating both device safety/performance and drug efficacy/stability within the delivery system, often extending the total time required for market approval compared to standalone drugs or devices.
Which therapeutic applications are currently driving the most significant growth in the HMN market?
The most significant growth drivers in the Hollow Microneedle market are high-value biologics delivery and vaccine administration. HMNs provide an ideal platform for delivering protein-based drugs, hormones (like insulin), and vaccines (e.g., flu, COVID-19), offering enhanced patient acceptance and potential logistical improvements, particularly for mass immunization campaigns globally, due to simplified administration protocols.
What are the main material types used in Hollow Microneedle fabrication, and why is material choice critical?
The main material types include silicon (for high precision and strength), metals (for durability), and polymers (for low-cost, flexibility, and biocompatibility, often dissolvable). Material choice is critical as it dictates the mechanical properties (ability to penetrate skin without breaking), manufacturing scalability, cost, and the overall functionality, particularly concerning the stability and release characteristics of the encapsulated drug formulation.
How is microfabrication technology impacting the scalability and cost-efficiency of Hollow Microneedle production?
Advanced microfabrication techniques, especially high-throughput methods like precision micro-molding and semiconductor-derived etching (DRIE), are dramatically improving the scalability and reducing the unit cost of Hollow Microneedle production. Automation and process optimization within cleanroom environments ensure consistent, high-quality arrays, transitioning HMNs from laboratory prototypes to commercially viable products capable of supporting large-scale pharmaceutical and vaccine markets globally.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
