Injectable Viscosupplementation Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 433613 | Date : Dec, 2025 | Pages : 248 | Region : Global | Publisher : MRU
Injectable Viscosupplementation Market Size
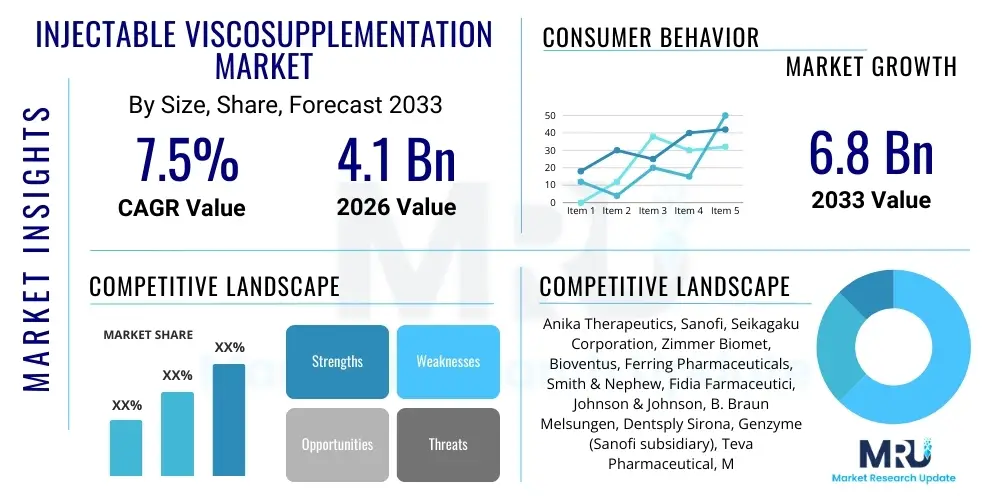
The Injectable Viscosupplementation Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.5% between 2026 and 2033. The market is estimated at USD 4.1 Billion in 2026 and is projected to reach USD 6.8 Billion by the end of the forecast period in 2033.
Injectable Viscosupplementation Market introduction
The Injectable Viscosupplementation Market encompasses the production and distribution of hyaluronic acid (HA) based therapies designed to treat joint pain, primarily associated with osteoarthritis (OA). Viscosupplementation involves injecting HA derivatives directly into the synovial fluid of affected joints, typically the knee, hip, or shoulder, to restore the lubricating and shock-absorbing properties lost due to cartilage degradation. This procedure offers a non-surgical, minimally invasive alternative for patients who have failed to respond adequately to conventional conservative treatments such as oral analgesics and physical therapy, providing significant pain relief and improving joint function and mobility.
The primary products in this market are categorized based on the concentration, molecular weight, and cross-linking structure of the hyaluronic acid utilized, leading to variations in treatment regimens, such as single-injection, three-injection, or five-injection courses. These products are critical in managing the symptoms of mild to moderate OA, delaying the need for total joint replacement surgery, especially in younger or highly active patient populations. Major applications center around large weight-bearing joints, with the knee osteoarthritis segment dominating due to high prevalence and established clinical guidelines supporting HA injection efficacy. The benefit profile of viscosupplementation includes localized action with reduced systemic side effects compared to NSAIDs, sustained pain relief often lasting six months or more, and functional improvement.
Key driving factors accelerating market growth include the rapidly aging global population, which correlates directly with an increasing prevalence of OA; heightened patient and physician awareness regarding the long-term side effects of chronic opioid and NSAID use; and continuous advancements in product technology, such as higher molecular weight, cross-linked formulations that permit single-dose administration, enhancing patient compliance and convenience. Furthermore, favorable reimbursement policies in developed economies and the growing adoption of minimally invasive procedures across emerging markets are substantial contributors to the sustained expansion of the injectable viscosupplementation therapeutic landscape.
Injectable Viscosupplementation Market Executive Summary
The Injectable Viscosupplementation Market is characterized by robust growth, driven primarily by the escalating incidence of osteoarthritis globally and the shift toward non-opioid pain management strategies. Business trends highlight intense competition centered on product differentiation, particularly the development of single-injection, highly cross-linked HA formulations that offer superior convenience and efficacy compared to traditional multi-dose regimens. Strategic collaborations, mergers, and acquisitions focused on expanding geographic reach and integrating novel drug delivery technologies are prominent features of the corporate landscape, aiming to capture increasing market share in the orthopedic and rheumatology sectors. Companies are also investing heavily in rigorous clinical trials to secure expanded indications for smaller joints, reinforcing their competitive positioning.
Regionally, North America maintains market dominance due to high healthcare expenditure, sophisticated reimbursement frameworks, and widespread acceptance of these therapies among orthopedic specialists, though the Asia Pacific (APAC) region is demonstrating the highest growth trajectory. This accelerated growth in APAC is attributable to improving healthcare infrastructure, increasing disposable incomes enabling access to advanced treatments, and a large, aging demographic base in countries like China and India. European markets, while mature, show stable growth, influenced heavily by regulatory approval processes and public healthcare coverage policies that dictate product uptake and price points. The Middle East and Africa (MEA) and Latin America represent emerging opportunities, contingent upon greater regulatory clarity and increased medical professional training regarding proper administration techniques.
Segment trends underscore the supremacy of the single-injection product category, which minimizes patient visits and administration burdens, thus attracting greater patient preference. Within applications, knee osteoarthritis remains the dominant segment; however, the hip and shoulder segments are projected to experience accelerated growth rates as clinical evidence for these off-label or newly approved applications strengthens. Furthermore, the Ambulatory Surgical Centers (ASCs) segment is rapidly increasing its market share among end-users, preferred for their efficiency and lower cost structures compared to traditional hospital settings, aligning with global trends toward outpatient specialized care delivery.
AI Impact Analysis on Injectable Viscosupplementation Market
Users frequently inquire about how Artificial Intelligence (AI) can enhance the efficacy, delivery, and personalization of injectable viscosupplementation treatments, alongside concerns regarding regulatory oversight and data security. The key themes revolve around AI's ability to predict optimal patient responders, refine injection techniques through augmented reality (AR) or robotics, and personalize the molecular composition of hyaluronic acid (HA) based on individual joint biomechanics. There is significant expectation that AI algorithms will analyze vast patient data—including imaging, biomarker panels, and clinical history—to determine the best product type (single vs. multi-injection) and timing for the treatment, moving the paradigm from reactive symptom management to proactive, personalized intervention. Concerns center on the cost of integrating these technologies into standard clinical practice and ensuring that AI-driven recommendations are clinically robust and accessible across diverse healthcare settings.
The integration of AI extends beyond clinical decision support into manufacturing and quality control. Machine learning models are being developed to optimize the fermentation and cross-linking processes of hyaluronic acid production, ensuring high purity, specific molecular weight distribution, and reduced batch variability, which directly impacts product safety and efficacy. Furthermore, predictive maintenance analytics powered by AI can monitor sophisticated manufacturing equipment, minimizing downtime and increasing yield, leading to overall cost efficiencies that can eventually translate into more affordable patient care. AI-enabled image analysis tools are also expected to improve the precision of diagnostic imaging used to assess the severity of osteoarthritis, providing more accurate grading that informs treatment planning for viscosupplementation.
In the realm of patient engagement and post-treatment monitoring, AI is utilized to develop sophisticated mobile applications and remote monitoring systems. These tools can track patient adherence, symptom progression, and functional recovery following the injection. Chatbots and virtual assistants offer personalized educational content and answer common patient queries, improving compliance and empowering patients in their recovery process. The resulting real-world evidence (RWE) collected via these AI platforms is crucial for payers and regulatory bodies, providing data on long-term treatment effectiveness outside controlled clinical trial environments, thereby reinforcing the value proposition of viscosupplementation products.
- AI-driven personalized treatment selection and dosage optimization based on individual patient biomarkers and severity of osteoarthritis.
- Enhanced injection precision using AI-powered robotic systems and augmented reality (AR) guidance for complex joint injections (e.g., hip).
- Machine learning optimization in HA manufacturing processes to ensure uniform molecular weight and high product purity.
- Predictive analytics for identifying non-responders early, thereby improving treatment effectiveness and resource allocation.
- Integration of AI algorithms for sophisticated analysis of MRI and X-ray images, providing more accurate grading of cartilage damage.
- Development of AI-enabled remote monitoring platforms for tracking patient outcomes, mobility improvement, and pain levels post-injection.
- Optimization of clinical trial design and patient recruitment efficiency through AI-based data analysis.
DRO & Impact Forces Of Injectable Viscosupplementation Market
The Injectable Viscosupplementation Market is propelled by robust drivers centered on demographic shifts and clinical demand, simultaneously facing significant restraints related to cost and competitive alternatives, balanced by compelling opportunities in product innovation and geographical expansion. The central impact force is the global rise in chronic joint conditions, particularly knee osteoarthritis, coupled with the urgent need for non-surgical interventions that mitigate the risks associated with long-term NSAID and opioid consumption. This force necessitates continuous innovation in HA formulation to enhance duration of effect and reduce injection frequency, creating a dynamic competitive environment where product efficacy and patient convenience dictate market success. The overall impact forces suggest a market poised for sustained, though perhaps incrementally challenged, expansion.
Drivers include the increasing geriatric population worldwide, leading directly to higher incidence rates of OA; growing patient demand for minimally invasive treatments over joint replacement surgery; and regulatory support for HA products as a proven, safe intervention. The escalating public health crisis surrounding opioid addiction further solidifies viscosupplementation’s role as an essential component of the orthopedic pain management pathway. Restraints largely encompass complex and sometimes inconsistent reimbursement policies across different geographies and healthcare systems, skepticism among some orthopedic surgeons regarding long-term efficacy compared to corticosteroids, and the high cost of treatment, particularly for premium, single-injection products. Competition from emerging regenerative therapies, such as Platelet-Rich Plasma (PRP) and cell-based treatments, also poses a constraint by offering alternative biological solutions.
Opportunities are abundant in developing novel, highly concentrated, or chemically modified HA derivatives that offer longer duration of action, potentially extending efficacy beyond 12 months, thereby addressing payer concerns regarding cost-effectiveness. Geographical expansion into underserved markets, especially in Asia and Latin America, where urbanization and lifestyle changes are driving up OA incidence, presents substantial growth potential. Furthermore, securing specific regulatory approvals for treating joints other than the knee (e.g., shoulder, hip, ankle, and temporomandibular joint) opens entirely new market segments. The strategic adoption of advanced delivery systems and combined therapies (e.g., HA combined with anti-inflammatory agents) will also unlock significant commercial value.
- Drivers: Rising global prevalence of osteoarthritis, increasing geriatric population base, growing preference for non-surgical pain management, clinical mandate to reduce opioid use, advancements in single-injection product technology.
- Restraints: High cost of treatment limiting patient access, variable and often limited insurance reimbursement coverage, competition from corticosteroid injections and emerging regenerative medicine (PRP, stem cells), temporary nature of pain relief necessitating repeat injections.
- Opportunities: Development of ultra-long-acting HA formulations, geographic expansion into high-growth emerging economies, new clinical approvals for non-knee joints, synergistic integration with physical therapy and rehabilitation programs, utilization of AI for patient selection.
- Impact Forces: Strong positive force from demographic aging and preference for minimal invasiveness, partially counteracted by pricing pressures and evolving competitive landscape.
Segmentation Analysis
The Injectable Viscosupplementation Market is systematically segmented based on product type, application site, and end-user, providing a granular view of market dynamics and adoption patterns. The segmentation by product type—single-injection, three-injection, and five-injection—is the most influential factor driving revenue distribution, reflecting the market’s consistent shift toward enhanced patient convenience and compliance offered by less frequent administration schedules. Single-injection products, utilizing highly cross-linked and high-molecular-weight HA, command premium pricing and are rapidly increasing their market share, often favored by both patients and busy clinicians seeking to minimize logistical overhead.
Analysis by application reveals that the treatment of knee osteoarthritis (OA) overwhelmingly dominates the market landscape. This dominance stems from the high incidence of knee OA, well-established clinical guidelines supporting the use of viscosupplementation, and extensive historical clinical trial data focused specifically on the knee joint. However, as evidence accumulates and awareness grows, the application segments for hip and shoulder OA treatment are exhibiting faster growth rates, indicating a future diversification of product usage across major and minor joints. This expansion is contingent upon regulatory agencies granting approvals for specific joints beyond the knee, validating the safety and efficacy in these diverse anatomical locations.
The end-user segmentation highlights the evolving landscape of healthcare delivery. While hospitals historically constituted the primary end-user segment due to the requirement for sterile environments and specialized medical personnel, Ambulatory Surgical Centers (ASCs) and Specialty Clinics are rapidly gaining prominence. ASCs are preferred for their cost-efficiency, streamlined processes, and dedicated focus on orthopedic outpatient procedures. Specialty clinics, particularly those focused on rheumatology and sports medicine, often act as crucial decision-makers and high-volume administrators of viscosupplementation treatments, impacting product selection and local market penetration strategies significantly.
- By Product Type:
- Single-injection Viscosupplements
- Three-injection Viscosupplements
- Five-injection Viscosupplements
- By Application:
- Knee Osteoarthritis
- Hip Osteoarthritis
- Shoulder Osteoarthritis
- Ankle and Other Joints Osteoarthritis
- By End-User:
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics and Orthopedic Centers
- Academic and Research Institutes
- By Source of Hyaluronic Acid:
- Animal-derived HA
- Non-animal derived/Bacterial Fermentation HA
Value Chain Analysis For Injectable Viscosupplementation Market
The value chain for the Injectable Viscosupplementation Market begins with the upstream sourcing and production of high-grade hyaluronic acid, primarily through bacterial fermentation or, less commonly today, extraction from avian sources. This crucial stage involves rigorous purification, precise molecular weight determination, and complex cross-linking processes required to engineer the desired viscosity and duration of effect for the final injectable product. Suppliers of raw materials, including specialized chemical manufacturers and fermentation specialists, hold significant power, as the quality and consistency of the HA precursor directly determines the therapeutic outcome and regulatory approval status of the final product. Vertical integration or exclusive long-term supply agreements are often used by major manufacturers to mitigate supply chain risks and ensure quality control.
The core manufacturing and midstream processes involve sophisticated sterile filling, quality assurance, and packaging into pre-filled syringes, optimizing for ease of administration in clinical settings. The downstream activities focus on distribution and marketing. Distribution channels are varied, including direct sales forces targeting orthopedic surgeons and rheumatologists in key accounts like major hospitals, as well as utilizing third-party medical device distributors and wholesalers to reach broader geographical areas and smaller clinics. The direct channel allows for closer relationship management and detailed product training, which is essential for complex injectable procedures, while indirect channels provide the necessary logistical reach and cost-effectiveness for high-volume sales. Effective marketing relies heavily on clinical evidence dissemination, physician education, and securing favorable reimbursement coding.
The market culminates at the point of end-user interaction: the administration of the injection by healthcare professionals (HCPs) to the patient. Key potential customers, including orthopedic surgeons, rheumatologists, and primary care physicians specializing in pain management, dictate market consumption. The efficiency of the distribution system—ensuring timely delivery and optimal inventory management to ASCs and clinics—is critical. Furthermore, the pharmaceutical and medical device companies invest substantially in post-market surveillance and patient follow-up programs, which feed back into research and development (R&D) to improve subsequent generations of viscosupplementation products, thus completing the cyclical value chain driven by clinical efficacy and patient outcomes.
Injectable Viscosupplementation Market Potential Customers
The primary potential customers and buyers within the Injectable Viscosupplementation Market are multifaceted, consisting of the institutions purchasing the product, the prescribing physicians, and the patients who ultimately receive the treatment. Institutional buyers primarily include major healthcare facilities such as large hospital systems that manage inpatient and outpatient orthopedic care, seeking high-volume discounts and standardized product lines for their multidisciplinary pain clinics. These systems prioritize products with strong clinical backing, favorable coverage from institutional payers, and established safety profiles, often integrating viscosupplementation into standardized care pathways for moderate osteoarthritis management before considering surgical options.
A rapidly expanding customer base is represented by Ambulatory Surgical Centers (ASCs) and specialized orthopedic or sports medicine clinics. ASCs act as crucial purchasing decision-makers, driven by the need for procedural efficiency and cost-effectiveness. They favor single-injection, easy-to-use products that maximize throughput and minimize inventory complexity. Individual orthopedic surgeons, rheumatologists, and pain management specialists are the key prescribers and administrators, whose adoption decisions are based on product efficacy, patient response rates, clinical experience, and the availability of reimbursement codes, influencing the choice of brand and dosage regimen.
Finally, the end-user—the patient suffering from chronic joint pain—exerts significant influence, particularly in healthcare systems where patients bear a portion of the cost. Patients seek treatments that offer sustained pain relief, improved mobility, and minimal disruption to their daily lives. They are increasingly informed consumers, researching product differences (e.g., duration, injection frequency) and often driving discussions with their physicians regarding specific brand preferences, especially in the context of avoiding or delaying invasive surgery. The market, therefore, requires a dual focus on appealing to both the clinical prescriber through efficacy data and the institutional purchaser through cost and operational benefits.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 4.1 Billion |
| Market Forecast in 2033 | USD 6.8 Billion |
| Growth Rate | CAGR 7.5% |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Anika Therapeutics, Sanofi, Seikagaku Corporation, Zimmer Biomet, Bioventus, Ferring Pharmaceuticals, Smith & Nephew, Fidia Farmaceutici, Johnson & Johnson, B. Braun Melsungen, Dentsply Sirona, Genzyme (Sanofi subsidiary), Teva Pharmaceutical, Merck KGaA, Flexion Therapeutics (Acquired by Pacira), Kaken Pharmaceutical, Lanius Biopharmaceuticals, Trice Medical, Shiseido Company, Lifecore Biomedical, Takeda Pharmaceutical Company. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Injectable Viscosupplementation Market Key Technology Landscape
The technological landscape of the Injectable Viscosupplementation Market is primarily defined by advanced materials science related to hyaluronic acid (HA) manipulation and sophisticated delivery systems. The most critical technological focus is on enhancing the residency time and efficacy of the injected material through specialized cross-linking and modification techniques. Cross-linking technologies, such as those utilizing divinyl sulfone (DVS) or 1,4-butanediol diglycidyl ether (BDDE), are employed to create highly viscous, stable gels that resist rapid enzymatic degradation within the joint, enabling the transition from multiple-injection protocols (e.g., traditional three or five shots) to highly favored single-injection products. Higher molecular weight and specific rheological properties are engineered to better mimic the healthy synovial fluid, maximizing lubrication and shock absorption.
Beyond material engineering, the market is increasingly adopting technologies that improve the precision and safety of the injection procedure. Ultrasound guidance technology is becoming a standard adjunct, particularly for non-knee joint injections like the hip and shoulder, ensuring accurate placement of the viscous material into the synovial space and reducing the risk of periarticular injection. Furthermore, pre-filled syringe technology is standardizing dose delivery, incorporating ergonomic design for easier handling by clinicians and often featuring safety mechanisms to prevent accidental needle stick injuries. These design optimizations are crucial for high-volume settings like ASCs, contributing significantly to patient safety and procedural efficiency.
An emerging technological frontier involves the exploration of combination therapies and novel drug delivery encapsulation. Manufacturers are investigating loading the HA matrix with adjunctive therapeutic agents, such as anti-inflammatory molecules (corticosteroids) or localized anesthetics, to provide immediate pain relief alongside the long-term cushioning effect of the HA. Micro- and nanoparticle encapsulation technologies are also being researched to control the sustained release of HA over a longer period, potentially creating products with efficacy extending beyond one year. These advancements leverage pharmaceutical expertise to transform the product from a simple mechanical lubricant into a sophisticated, active pharmaceutical delivery system, addressing the market need for longer-lasting, more comprehensive OA management solutions.
Regional Highlights
Regional dynamics within the Injectable Viscosupplementation Market reveal distinct patterns of adoption, driven by healthcare structure, aging demographics, and reimbursement policies, necessitating tailored market penetration strategies for global manufacturers.
- North America (U.S. and Canada): Dominates the global market share, characterized by high disposable incomes, advanced healthcare infrastructure, and extensive insurance coverage for orthopedic procedures. The U.S. drives innovation and early adoption of premium, single-injection viscosupplements. Market growth is stable, underpinned by a high prevalence of OA and strong clinical acceptance among specialists.
- Europe: Represents a mature market with robust demand, heavily influenced by country-specific public and private reimbursement systems. Germany, France, and the UK are key contributors. Growth is consistent, propelled by an aging population, though price sensitivity is higher compared to the U.S. due to national healthcare budget constraints.
- Asia Pacific (APAC): Exhibits the fastest Compound Annual Growth Rate (CAGR). This acceleration is fueled by the massive patient pool, rapid improvements in healthcare access (especially in China and India), increasing awareness of non-surgical OA treatments, and rising medical tourism. Regulatory hurdles, while present, are being streamlined, paving the way for substantial commercial expansion.
- Latin America (LATAM): A developing market characterized by fragmented healthcare systems and variable economic stability. Growth is driven primarily by private healthcare sectors in countries like Brazil and Mexico. The challenge remains achieving broader accessibility and securing favorable public sector procurement.
- Middle East and Africa (MEA): Currently holds the smallest share but offers niche opportunities in wealthy Gulf Cooperation Council (GCC) countries (e.g., UAE, Saudi Arabia) which possess world-class specialized orthopedic centers. Market penetration is slow in Sub-Saharan Africa due to low medical infrastructure development and high procedure costs relative to local incomes.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Injectable Viscosupplementation Market.- Anika Therapeutics, Inc.
- Sanofi S.A.
- Seikagaku Corporation
- Zimmer Biomet Holdings, Inc.
- Bioventus LLC
- Ferring Pharmaceuticals S.A.
- Smith & Nephew plc
- Fidia Farmaceutici S.p.A.
- Johnson & Johnson (DePuy Synthes)
- B. Braun Melsungen AG
- Dentsply Sirona Inc.
- Genzyme (a subsidiary of Sanofi)
- Teva Pharmaceutical Industries Ltd.
- Merck KGaA
- Flexion Therapeutics (Acquired by Pacira BioSciences, Inc.)
- Kaken Pharmaceutical Co., Ltd.
- Lanius Biopharmaceuticals
- Trice Medical
- Shiseido Company, Limited
- Lifecore Biomedical, Inc.
Frequently Asked Questions
Analyze common user questions about the Injectable Viscosupplementation market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary factor driving the current growth of the Injectable Viscosupplementation Market?
The principal driver is the globally increasing prevalence of osteoarthritis (OA), particularly among the rapidly expanding geriatric population, coupled with the healthcare sector’s critical need for effective, non-surgical alternatives that minimize reliance on long-term opioid and NSAID use for pain management.
Which product segment, single-injection or multi-injection, dominates the market share and why?
The single-injection viscosupplement segment is gaining dominance and holds significant revenue share. This preference is driven by enhanced patient convenience and compliance, as it reduces the required number of clinical visits and administration burden compared to the traditional three- or five-injection regimens.
What major restraints challenge the widespread adoption of injectable viscosupplementation therapies?
Key challenges include the high cost of premium viscosupplement products, which often leads to inconsistent or limited reimbursement coverage across various national and private healthcare plans, alongside growing competitive pressure from emerging regenerative therapies like Platelet-Rich Plasma (PRP).
How is technology impacting the development of new viscosupplementation products?
Technology is focused on advanced molecular engineering, specifically specialized cross-linking of hyaluronic acid (HA) to increase its molecular weight and residency time within the joint, creating longer-lasting, more effective single-dose treatments. Additionally, ultrasound guidance and pre-filled syringe systems are enhancing procedural safety and accuracy.
Which geographical region is expected to exhibit the fastest growth in this market through 2033?
The Asia Pacific (APAC) region is projected to register the highest Compound Annual Growth Rate (CAGR). This rapid expansion is attributed to demographic aging, improvements in regional healthcare infrastructure, rising disposable incomes facilitating access to advanced treatments, and a large, untapped patient population base.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
