Japanese Encephalitis Vaccine Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 437157 | Date : Dec, 2025 | Pages : 257 | Region : Global | Publisher : MRU
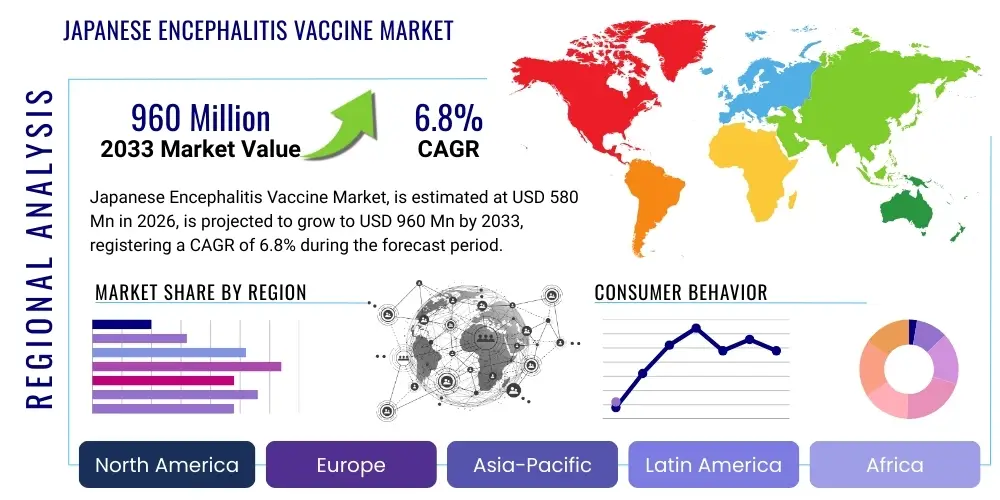
Japanese Encephalitis Vaccine Market Size
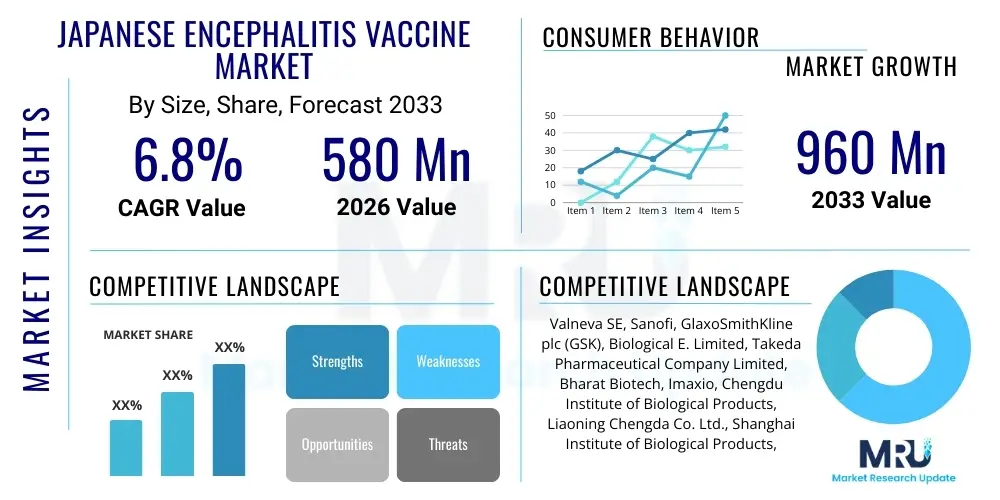
The Japanese Encephalitis Vaccine Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2026 and 2033. The market is estimated at USD 580 Million in 2026 and is projected to reach USD 960 Million by the end of the forecast period in 2033.
Japanese Encephalitis Vaccine Market introduction
The Japanese Encephalitis (JE) Vaccine Market encompasses the global commercialization, distribution, and utilization of prophylactic treatments designed to prevent infection by the Japanese Encephalitis virus, a mosquito-borne flavivirus endemic to numerous regions in Asia and the Western Pacific. This market is fundamentally driven by high disease prevalence in endemic zones, increasing government-led immunization programs, and the rising awareness concerning travel medicine and mandatory vaccination protocols for international travelers visiting high-risk areas. The development landscape includes various vaccine technologies such as inactivated, live attenuated, and recombinant subunit vaccines, each offering distinct advantages in terms of efficacy, safety profiles, and cost-effectiveness for different population segments and national immunization strategies. Continuous research is focused on developing thermostable and single-dose options to enhance deployment efficiency in remote or resource-limited settings.
Major applications for JE vaccines are broadly categorized into routine pediatric immunization programs, ensuring protection for children residing in hyper-endemic areas where transmission cycles are persistent, and immunization for travelers, military personnel, and expatriates moving into or within endemic zones. The primary benefits of these vaccines include significant reduction in morbidity and mortality associated with JE, a disease known for causing severe neuroinvasive syndromes that often result in long-term neurological sequelae or death. Successful vaccination campaigns are pivotal in alleviating the healthcare burden on national systems in countries like India, China, and Vietnam, where seasonal outbreaks pose significant public health challenges.
Driving factors propelling market expansion include enhanced funding from global health organizations such as the WHO and Gavi, which support large-scale vaccine procurement and distribution in developing countries. Furthermore, shifts in climate patterns affecting mosquito vectors are expanding the geographical reach of JE incidence, necessitating broader vaccination coverage. Technological advancements, particularly in cell-culture-derived vaccines (like those manufactured using Vero cell lines), are improving manufacturing scalability and purity, ensuring a reliable supply chain. However, market growth is often restrained by the high cost of newer generation vaccines in certain regions and logistical hurdles related to maintaining cold chains in tropical environments. The continuous vigilance of public health agencies and the integration of JE vaccination into national Essential Immunization Schedules remain critical for sustained market growth and disease control.
Japanese Encephalitis Vaccine Market Executive Summary
The Japanese Encephalitis Vaccine Market is poised for robust expansion, primarily fueled by aggressive public health initiatives targeting pediatric populations in high-incidence Asian nations and increasing global travel exposure. Business trends indicate a strategic pivot toward cell-culture-derived inactivated and recombinant vaccines, which are gradually replacing older, mouse brain-derived variants due to superior safety profiles and ease of manufacturing scale-up. Key pharmaceutical manufacturers are engaging in strong public-private partnerships, collaborating with governmental and non-governmental organizations to facilitate large volume tenders and ensure vaccine affordability and accessibility across endemic areas. Innovation focuses heavily on reducing the required dosage regimen and improving thermal stability to optimize logistics, thereby mitigating the restraints imposed by complex cold chain requirements in tropical climates. Furthermore, the market is characterized by consolidation among top-tier players acquiring smaller specialized biotech firms to secure novel production technologies and expand regional manufacturing footprints.
Regionally, the Asia Pacific segment dominates the market, contributing the largest share of revenue and volume due to the endemic nature of the disease in densely populated nations such as China, India, and Southeast Asia. These regions host the primary target population for mass vaccination campaigns. However, developed regions like North America and Europe demonstrate significant growth in the travel vaccine segment, driven by heightened health awareness among international travelers and regulatory requirements for pre-travel consultation. The implementation of robust surveillance systems and increased diagnostic capacity in emerging markets further strengthens the demand for prophylactic JE immunization. Strategic market entrants are focusing on market access strategies, including obtaining WHO prequalification, which is crucial for participation in international tenders funded by multilateral organizations.
Segment trends highlight the dominance of inactivated vaccines, particularly the Vero cell-derived variants, owing to their proven efficacy and favorable regulatory approval status in multiple geographies. The pediatric segment remains the primary application area, generating the highest demand volume as numerous countries integrate JE vaccines into their national immunization schedules for infants and young children. However, the adult segment is experiencing accelerated growth, largely driven by occupational exposure (e.g., agricultural workers, researchers) and travel-related vaccination requirements. Distribution channels are shifting, with greater reliance on government procurement agencies and institutional tenders, ensuring centralized purchasing power and coordinated delivery to target demographics, although private distribution via specialized travel clinics remains important in non-endemic regions.
AI Impact Analysis on Japanese Encephalitis Vaccine Market
Analysis of common user questions regarding the influence of Artificial Intelligence (AI) on the Japanese Encephalitis (JE) vaccine market reveals significant curiosity and high expectations concerning accelerated R&D, enhanced outbreak prediction, and optimization of manufacturing processes. Users frequently inquire about how AI can expedite novel vaccine design, specifically in identifying new epitopes or developing thermostable formulations. Concerns often revolve around the ethical deployment of AI in resource allocation during outbreaks and the reliability of AI-driven epidemiological models. Key themes consistently emerging include the use of machine learning for personalized risk assessment for travelers, optimizing the cold chain logistics using predictive algorithms, and improving clinical trial efficiency by identifying ideal cohort populations in endemic regions. Users anticipate that AI integration will fundamentally shorten the vaccine development lifecycle and significantly improve the efficacy of public health responses to JE outbreaks, thereby driving market responsiveness and reducing disease burden.
- AI optimizes vaccine design by modeling viral protein structures and predicting ideal antigenic targets, potentially leading to faster development of highly effective recombinant vaccines.
- Machine learning algorithms enhance epidemiological surveillance by analyzing environmental, climate, and demographic data to predict JE outbreak hotspots and seasonality with greater accuracy, aiding preemptive vaccination deployment.
- AI-driven supply chain management systems improve cold chain logistics, predicting temperature deviations and optimizing transportation routes, ensuring vaccine potency upon delivery to remote areas.
- Generative AI supports the acceleration of preclinical research by simulating complex biological interactions and screening potential adjuvants, reducing time and cost in the discovery phase.
- AI models assist in analyzing large volumes of post-market surveillance data, identifying rare adverse events more quickly and contributing to enhanced vaccine safety monitoring and regulatory compliance.
DRO & Impact Forces Of Japanese Encephalitis Vaccine Market
The market dynamics of the Japanese Encephalitis Vaccine sector are powerfully shaped by an interplay of substantial drivers, critical restraints, and emerging opportunities, all unified under significant impact forces that determine the trajectory of growth and market accessibility. The primary driver is the mandated inclusion of JE vaccination in National Immunization Programs (NIPs) across endemic countries, supported heavily by global health funding bodies. Counteracting this momentum are significant restraints, primarily the high cost of next-generation cell-culture vaccines and the operational challenges inherent in maintaining strict cold chain requirements across vast and often underdeveloped distribution networks. Opportunities arise from expanding endemic zones due to climate change, leading to new geographical markets, and the push for single-dose, needle-free alternatives that would revolutionize mass immunization campaigns.
Impact forces are multifaceted, ranging from technological advancements to intense regulatory scrutiny. The development of thermostable formulations, for instance, acts as a significant positive impact force, reducing logistical complexity and costs associated with distribution. Conversely, the public perception of vaccine safety, influenced by localized misinformation or previous adverse event scares, acts as a negative restraining force that can momentarily halt or slow down mass immunization uptake, particularly in newly targeted regions. The competitive landscape is also a key impact force; the presence of high-volume, low-cost manufacturers, predominantly in Asia, maintains downward pressure on pricing, which, while beneficial for procurement agencies, limits profit margins for innovator companies, driving them toward premium recombinant products for travelers' markets.
Ultimately, the market’s responsiveness is contingent upon collaborative governmental and corporate efforts. Effective political will to enforce NIPs and adequate financial provisioning for procurement tenders directly amplify the market’s growth drivers. The continuous investment in R&D to address the need for broader cross-protection against different JE viral genotypes further solidifies the market's long-term sustainability. Overcoming the financial barrier for low-income countries through differential pricing strategies and technology transfer partnerships remains central to converting potential market opportunity into realized revenue growth and successfully curbing the global JE burden.
- Drivers:
- Mandatory integration of JE vaccines into routine childhood immunization schedules in high-incidence countries.
- Increased funding and support from global health organizations (e.g., Gavi, WHO) for procurement and distribution.
- Rising incidence of travel to endemic regions, boosting the demand for travel medicine prophylaxis in non-endemic countries.
- Expansion of the geographical endemic zones due to global climate change affecting mosquito vector habitats.
- Restraints:
- Significant challenges in maintaining the cold chain logistics required for vaccine stability, particularly in rural and tropical areas.
- High manufacturing costs associated with modern cell-culture-derived vaccines compared to older generations.
- Hesitancy or resistance towards mass vaccination programs fueled by public skepticism or misinformation campaigns.
- Regulatory complexities and long approval timelines for new vaccine formulations entering diverse national markets.
- Opportunity:
- Development and commercialization of next-generation vaccines, including single-dose and oral/nasal delivery systems, improving patient compliance.
- Targeting large, previously underserved populations in newly identified JE endemic or epidemic-prone areas.
- Strategic partnerships between global manufacturers and local production hubs to ensure regional self-sufficiency and lower costs.
- Advancement in combination vaccines that protect against multiple mosquito-borne diseases alongside JE.
- Impact Forces:
- Technological advancements in thermostability and formulation efficacy.
- Governmental purchasing policies and central tender processes.
- Global surveillance effectiveness and rapid response mechanisms.
- Competitive pricing pressure from Asian manufacturers.
Segmentation Analysis
The Japanese Encephalitis Vaccine Market is systematically segmented based on Product Type, Application, End-User, and Distribution Channel, allowing for nuanced strategic planning and detailed market sizing tailored to specific demographic and regional needs. Understanding these segments is crucial for manufacturers to align their production capabilities and commercialization strategies with prevailing demand patterns. For instance, the transition from older mouse brain-derived vaccines to modern cell-culture and recombinant types represents the most significant shift within the Product Type segment, reflecting global preferences for improved safety profiles and reduced reactogenicity. Market participants must carefully analyze the purchasing power and regulatory environments within each segment to maximize penetration and profitability, particularly balancing high-volume, low-margin institutional sales against lower-volume, high-margin private travel clinic sales.
The Application segmentation distinguishes between pediatric immunization, which is generally part of routine schedules, and adult vaccination, often driven by occupational risk or travel itineraries. This division dictates marketing focus and educational materials; pediatric campaigns focus on herd immunity and community health, whereas adult campaigns emphasize individual protection and risk management. End-User segmentation, encompassing governmental bodies, hospitals, and NGOs, further clarifies the procurement landscape, indicating the need for specific tender management capabilities versus direct sales expertise. Furthermore, distribution channel analysis highlights the dominance of governmental procurement agencies in endemic regions, acting as central purchasing bodies, contrasting sharply with the reliance on specialized retail and travel pharmacies in non-endemic, high-income markets.
- By Product Type: Inactivated Vaccines (Cell-Culture Derived), Live Attenuated Vaccines, Recombinant Subunit Vaccines, Mouse Brain-Derived Vaccines (Phase-out/Legacy).
- By Application: Pediatric Vaccination (Routine Immunization), Adult Vaccination (Travelers, Occupational Exposure, Military).
- By End-User: Hospitals and Clinics, Government Organizations and Public Health Agencies, Non-Governmental Organizations (NGOs) and International Aid Bodies, Research Institutions.
- By Distribution Channel: Government Supply & Institutional Sales (Tenders), Retail Pharmacies and Drug Stores, Online Pharmacies, Specialized Travel Clinics.
Value Chain Analysis For Japanese Encephalitis Vaccine Market
The value chain for the Japanese Encephalitis Vaccine Market begins with upstream activities focused on sophisticated research and development, involving gene sequencing, cell line development (Vero cells), and antigen purification protocols. This initial phase requires substantial intellectual capital and investment in specialized biomanufacturing facilities capable of meeting Good Manufacturing Practice (GMP) standards. Raw material sourcing involves procuring high-quality biological media, adjuvants, and stabilizers, often from specialized global suppliers. Key players focus intensely on optimizing upstream yield and stability, as these factors directly determine the scalability and cost-effectiveness of the final product. Licensing and intellectual property management are also crucial upstream components, especially for advanced recombinant technologies which often involve multi-party agreements and complex patent portfolios.
The central manufacturing stage includes large-scale fermentation or cell culture, purification, formulation, and aseptic filling, followed by meticulous quality control (QC) and assurance (QA). Midstream efficiency, particularly the capacity for high-volume production, is vital for companies targeting large government tenders. Downstream activities involve packaging, labeling, and specialized distribution logistics, crucially relying on a reliable cold chain from the manufacturing site to the point of administration. The distribution channel is often bifurcated: direct sales via centralized government tenders ensure efficient mass deployment in endemic areas, while indirect channels leverage wholesale distributors, specialized travel clinics, and retail pharmacies in regions where JE vaccination is discretionary or for travel purposes. The successful navigation of stringent international and national regulatory approval processes is the final, critical downstream component ensuring market access.
The complexities of the cold chain, necessitating specialized refrigerated transport and storage (2°C to 8°C), significantly influence the distribution costs and network structure. Direct governmental procurement channels often bypass multiple intermediaries, ensuring cost efficiency and quicker delivery to public health centers, which is particularly vital during outbreak situations. Conversely, the indirect channel in developed nations provides convenience and personalized advice through healthcare providers specializing in travel medicine, justifying the higher price points observed in these markets. Optimizing the flow of information and product integrity across both channels requires robust inventory management and track-and-trace systems, increasingly utilizing advanced digital solutions to monitor environmental conditions throughout transit and storage, ultimately reinforcing the vaccine’s safety and efficacy upon final administration.
Japanese Encephalitis Vaccine Market Potential Customers
The potential customer base for the Japanese Encephalitis Vaccine Market is highly diverse, spanning both public health institutions focused on mass protection and individual consumers seeking personal prophylaxis. The largest volume buyers are national governments in Asia Pacific countries, including China, India, Indonesia, and Vietnam, who procure vaccines in bulk through global tenders for mandatory inclusion in their national pediatric immunization programs (NIPs). These governmental agencies act as the primary end-users, responsible for disease prevention across millions of citizens, and their purchasing decisions are largely driven by WHO prequalification status, cost-effectiveness, and proven public health impact. Furthermore, international non-governmental organizations (NGOs) like Médecins Sans Frontières (MSF) and international development agencies often purchase vaccines for emergency responses or immunization campaigns in underserved, high-risk territories.
A rapidly expanding customer segment includes adults categorized as travelers, military personnel, and expatriates. Travelers from non-endemic countries (e.g., North America and Europe) visiting rural areas of Asia represent a high-value customer base for private healthcare providers and travel medicine clinics. These customers prioritize convenience, safety, and brand reputation, often opting for premium, newer-generation vaccines. Military forces deployed to high-risk areas globally constitute another specific institutional buyer segment, demanding highly efficacious and reliable vaccines for personnel protection. In endemic regions, potential customers also include healthcare workers and veterinary professionals exposed to the virus via infected animals, although this volume is typically smaller than the mass immunization programs.
The shift in customer focus necessitates flexible manufacturing strategies. Manufacturers must be able to produce the high-volume, low-cost vaccines demanded by governmental organizations while simultaneously maintaining supply chains for the lower-volume, premium products favored by private travel clinics in the West. Ultimately, the end-user remains the vaccinated individual—the infant protected through a national program or the adult traveler seeking precautionary measures—but the purchasing decision power resides predominantly with large institutional buyers and specialist medical practitioners who recommend and administer the prophylactic treatment based on geographical risk assessment and individual health profiles.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 580 Million |
| Market Forecast in 2033 | USD 960 Million |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Valneva SE, Sanofi, GlaxoSmithKline plc (GSK), Biological E. Limited, Takeda Pharmaceutical Company Limited, Bharat Biotech, Imaxio, Chengdu Institute of Biological Products, Liaoning Chengda Co. Ltd., Shanghai Institute of Biological Products, Panacea Biotec, Green Cross Corporation, Crucell N.V. (J&J), Merck & Co., Inc., Serum Institute of India Pvt. Ltd., Xiamen Amoytop Biotech Co., Ltd., Biken Co., Ltd., Sinovac Biotech Ltd., Haffkine Bio-Pharmaceutical Corporation Ltd. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Japanese Encephalitis Vaccine Market Key Technology Landscape
The technological landscape of the Japanese Encephalitis (JE) Vaccine Market is characterized by a significant transition away from legacy, first-generation mouse brain-derived vaccines towards modern, safer, and more scalable production platforms. The most prevalent technology driving current market supply is the cell-culture based inactivation method, typically utilizing Vero cell lines. This technology offers high purity, consistent potency, and significantly reduced risk of neurological side effects compared to older methods, thereby improving patient acceptance and meeting stringent international regulatory standards, including WHO prequalification. Key advancements focus on optimizing the cell culture media and harvesting processes to maximize antigen yield per batch, leading to improved manufacturing economics and a more reliable global supply to meet the demands of large-scale national immunization programs.
A second crucial technological vector involves the development and deployment of live attenuated vaccines, which offer the advantage of eliciting a robust and long-lasting immune response often requiring only a single dose. These vaccines, exemplified by the SA 14-14-2 strain, are particularly cost-effective and highly utilized in mass campaigns across specific Asian countries. However, the current frontier of innovation lies in recombinant and subunit vaccine technologies. These next-generation vaccines utilize genetic engineering to produce specific viral envelope proteins (like the JE E protein) in expression systems such as yeast or insect cells. Recombinant technology promises even higher safety profiles, the potential for thermostability, and the capacity for rapid modifications if the dominant circulating viral genotypes shift, addressing the long-term threat of viral evolution and vaccine escape.
Furthermore, ongoing research is exploring novel delivery mechanisms and formulation improvements. This includes investigating adjuvants that can enhance the immune response, reducing the necessary dosage and overall production cost. The implementation of stabilization technologies aimed at creating lyophilized, thermostable formulations is a major strategic priority. A thermostable vaccine would significantly mitigate the major constraint of cold chain requirements in tropical environments, potentially revolutionizing distribution efficiency in rural Asian and African markets. The integration of high-throughput screening and analytical technologies also plays a critical role in quality control, ensuring batch consistency and expedited regulatory filing for market expansion.
Regional Highlights
The geographical analysis of the Japanese Encephalitis Vaccine Market reveals distinct consumption and growth patterns driven by disease endemicity, public health policies, and economic development levels across the globe. Asia Pacific (APAC) stands as the undisputed market leader, both in terms of volume and value, largely because JE is hyper-endemic across vast swathes of South and Southeast Asia. Countries like China, India, Vietnam, and Thailand have implemented aggressive, government-funded mass vaccination programs for children, constituting the largest segment of global demand. High disease incidence and large population bases ensure continued dominance, though competitive pricing pressure is intense due to the presence of multiple local, high-volume manufacturers.
North America and Europe, while being non-endemic zones, represent the fastest-growing segments in terms of revenue per dose, driven entirely by the demand for travel vaccines. The increasing rate of international tourism, business travel, and military deployment into JE-endemic regions fuels robust demand for premium, highly approved vaccines (e.g., IXIARO/JE-VC). This market segment is characterized by higher pricing, specialized distribution through travel clinics, and strong reliance on health awareness campaigns and physician recommendations, making it a high-margin opportunity for multinational corporations.
Latin America and the Middle East & Africa (MEA) currently hold smaller market shares, primarily because JE is not traditionally endemic in these regions. However, market potential exists in areas where mosquito vectors or potential reservoirs might overlap or expand due to climate shifts. Additionally, MEA plays a vital role as a recipient of global aid and emergency vaccine supplies, particularly through NGO-led initiatives. As surveillance capabilities improve and global health agencies advocate for preparedness against emerging infectious diseases, the strategic importance of ensuring vaccine availability in these regions, even as a precautionary measure, is projected to increase, opening new, albeit smaller, institutional procurement opportunities.
- Asia Pacific (APAC): Dominant market share due to endemic status, extensive government-led pediatric immunization programs, and high patient volume in countries like India, China, and Vietnam. Characterized by high-volume, cost-sensitive institutional tenders.
- North America: Significant growth in the travel vaccine segment, driven by high disposable incomes and strong awareness of travel health risks. Demand focuses on premium, cell-culture derived vaccines (JE-VC).
- Europe: Similar to North America, growth is concentrated in the adult travel market, supported by established travel health infrastructure and mandatory pre-travel consultation services.
- Latin America: Minimal current market presence, but serves as a potential growth area driven by increasing surveillance and precautionary measures against geographically expanding arboviruses.
- Middle East and Africa (MEA): Relies heavily on international aid and global tenders for sporadic or precautionary vaccination campaigns, representing potential for future market entry as surveillance improves.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Japanese Encephalitis Vaccine Market.- Valneva SE
- Sanofi
- GlaxoSmithKline plc (GSK)
- Biological E. Limited
- Takeda Pharmaceutical Company Limited
- Bharat Biotech
- Imaxio
- Chengdu Institute of Biological Products
- Liaoning Chengda Co. Ltd.
- Shanghai Institute of Biological Products
- Panacea Biotec
- Green Cross Corporation
- Crucell N.V. (A Johnson & Johnson Company)
- Merck & Co., Inc.
- Serum Institute of India Pvt. Ltd.
- Xiamen Amoytop Biotech Co., Ltd.
- Biken Co., Ltd.
- Sinovac Biotech Ltd.
- Haffkine Bio-Pharmaceutical Corporation Ltd.
Frequently Asked Questions
Analyze common user questions about the Japanese Encephalitis Vaccine market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the projected growth rate (CAGR) for the Japanese Encephalitis Vaccine Market?
The Japanese Encephalitis Vaccine Market is projected to exhibit a steady Compound Annual Growth Rate (CAGR) of approximately 6.8% during the forecast period from 2026 to 2033, driven primarily by expanded immunization programs in Asia Pacific.
Which vaccine product type currently holds the largest market share in the JE segment?
Inactivated vaccines, particularly those derived from Vero cell culture (like JE-VC), hold the largest market share due to their superior safety profile, efficacy, and widespread adoption in governmental procurement and traveler immunization markets globally.
Which geographical region dominates the demand for Japanese Encephalitis vaccines?
The Asia Pacific region dominates the global market demand by volume, as it encompasses the endemic zones with the highest burden of disease and mandates large-scale pediatric immunization programs across densely populated nations like India and China.
What is the primary constraint hindering market growth in developing countries?
The primary constraint is the significant logistical challenge and high operational cost associated with maintaining the strict cold chain requirements (2°C to 8°C) necessary to preserve vaccine potency, particularly in remote or tropical endemic areas.
How is AI expected to influence the future of Japanese Encephalitis vaccine development?
AI is expected to accelerate R&D by predicting optimal antigenic targets and modeling viral behavior, and to enhance market efficiency by optimizing cold chain logistics and improving the accuracy of outbreak surveillance and forecasting.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager