Lateral Flow Assay Sales Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 432983 | Date : Dec, 2025 | Pages : 258 | Region : Global | Publisher : MRU
Lateral Flow Assay Sales Market Size
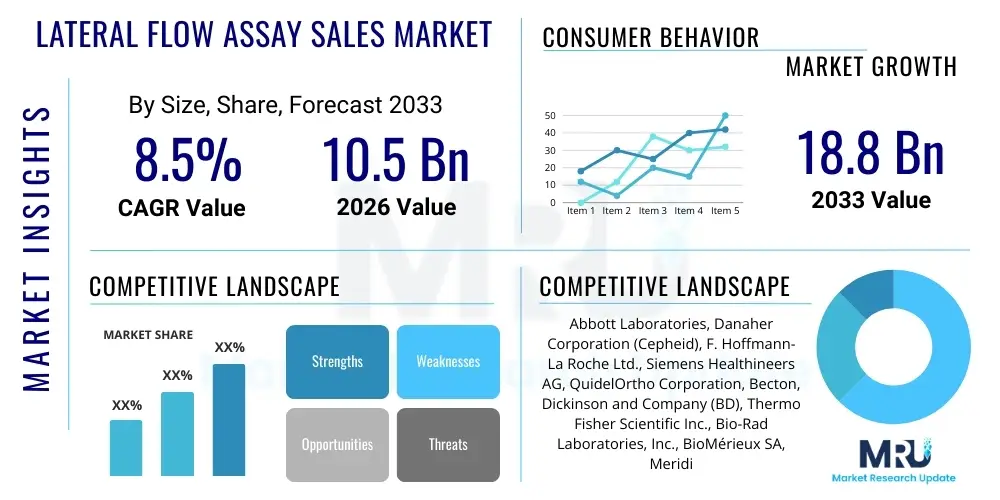
The Lateral Flow Assay Sales Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.5% between 2026 and 2033. The market is estimated at USD 10.5 Billion in 2026 and is projected to reach USD 18.8 Billion by the end of the forecast period in 2033.
Lateral Flow Assay Sales Market introduction
The Lateral Flow Assay (LFA) Sales Market encompasses the global trade of simple, rapid, and cost-effective diagnostic devices designed for point-of-care testing. These assays function based on the principle of liquid migration through a porous membrane, typically utilizing antibodies to detect specific target analytes, such as proteins, hormones, or infectious agents, in complex matrices like blood, urine, or saliva. The key products include test strips, cartridges, and integrated reader systems, crucial for generating qualitative or semi-quantitative results quickly without requiring sophisticated laboratory infrastructure. LFAs are widely used across various medical and non-medical fields due to their user-friendliness and rapid turnaround time, making them indispensable tools for decentralized testing globally.
Major applications driving the demand in this market include rapid diagnosis of infectious diseases, such as COVID-19, influenza, and HIV, alongside critical applications in pregnancy testing, toxicology (drugs of abuse screening), and monitoring chronic conditions. The primary benefit of adopting LFAs lies in their capacity to deliver immediate results at the point of need, significantly enhancing patient management, especially in resource-limited settings or during large-scale public health crises. This immediacy reduces the time required for diagnosis and initiation of treatment, which is vital for controlling outbreaks and improving health outcomes globally. Furthermore, the simplicity of the test procedure minimizes the need for highly trained personnel, broadening their accessibility across diverse healthcare environments.
The market is significantly driven by the increasing global prevalence of infectious diseases, the growing emphasis on early diagnosis, and the rising demand for decentralized testing solutions (Point-of-Care Testing, POCT). Technological advancements, including the incorporation of nanotechnology and digital reader systems to improve sensitivity and quantification capabilities, are further accelerating market expansion. Additionally, regulatory support for home-use diagnostics and the continuous development of novel biomarker panels tailored for LFA platforms are providing substantial growth impetus, ensuring sustained high-volume sales of both assay kits and reader devices across developed and emerging economies.
Lateral Flow Assay Sales Market Executive Summary
The Lateral Flow Assay Sales Market is experiencing robust expansion driven primarily by the persistent need for rapid diagnostics, particularly following major global health emergencies that underscored the necessity of decentralized testing infrastructure. Current business trends indicate a strong shift towards developing quantitative LFA platforms, moving beyond traditional qualitative results, which is enhancing their utility in clinical settings and creating premium pricing opportunities. Strategic mergers, acquisitions, and partnerships aimed at expanding geographical reach and integrating advanced sensor technologies are characteristic of the competitive landscape, focused on achieving higher sensitivity and specificity to compete with laboratory-based techniques. Furthermore, there is a pronounced push toward regulatory harmonization, which facilitates quicker market entry for novel assay designs globally, supporting increased sales volumes.
Regionally, North America and Europe maintain dominance owing to high healthcare expenditure, established POCT infrastructure, and the early adoption of advanced diagnostic technologies, including digital readers and multiplex LFAs. However, the Asia Pacific (APAC) region is projected to register the highest Compound Annual Growth Rate (CAGR), fueled by massive populations, increasing awareness of infectious disease screening, governmental initiatives promoting accessible healthcare, and the rapid expansion of diagnostic laboratories and primary care facilities in countries like China and India. Emerging economies in Latin America and the Middle East & Africa (MEA) are also showing promising growth, primarily driven by international aid programs and local efforts to combat prevalent endemic diseases using cost-effective LFA solutions.
Segmentation trends highlight the dominance of infectious disease testing applications, especially those targeting respiratory viruses and sexually transmitted infections, which continue to drive the highest volume of LFA sales. Within product segments, assay kits and reagents constitute the largest revenue share, reflecting the consumable nature of the products, while the reader systems segment is poised for rapid growth due to the integration of connectivity and artificial intelligence (AI) for automated result interpretation and data management. End-user demand remains highest in hospitals and diagnostic laboratories, but the fastest growing segment is home-use settings, catalyzed by the increasing acceptance and regulatory approval of self-testing kits for conditions ranging from fertility monitoring to screening for common pathogens.
AI Impact Analysis on Lateral Flow Assay Sales Market
User inquiries regarding the impact of Artificial Intelligence (AI) on the Lateral Flow Assay (LFA) market primarily revolve around three core themes: improving diagnostic accuracy, automating interpretation and reducing human error, and integrating LFA results into larger digital health ecosystems. Users frequently ask how AI can enhance the sensitivity of reader-based LFAs, allowing them to detect lower concentrations of analytes, which is a major limitation of conventional visual reading. Concerns are also raised about the standardization and validation processes required for AI algorithms used in regulatory submissions for LFA devices. A high level of expectation exists regarding AI's role in connecting rapid testing results directly to electronic health records (EHRs) and epidemiological surveillance systems, thereby transforming rapid testing from a standalone diagnostic event into an integrated component of precision medicine and public health monitoring.
- AI-powered image analysis algorithms enhance the objectivity and precision of LFA result interpretation, reducing inter-observer variability inherent in manual reading.
- Machine learning models optimize the calibration and quality control of digital LFA readers, ensuring consistent performance across various environmental conditions.
- Integration of AI facilitates automated data logging and real-time transmission of results from point-of-care settings to centralized laboratory information systems (LIS) or public health databases.
- Predictive AI analytics can correlate LFA results with patient metadata to assist clinicians in initial differential diagnosis, particularly in resource-constrained environments.
- AI assists in the design and optimization of novel LFA strip components and chemistry by simulating flow dynamics and binding kinetics, accelerating product development cycles.
- Enhanced pattern recognition through deep learning allows for the simultaneous interpretation of multiplex LFA results, increasing the information yield from a single test.
DRO & Impact Forces Of Lateral Flow Assay Sales Market
The Lateral Flow Assay Sales Market is fundamentally shaped by a delicate balance between the urgent global demand for rapid diagnostics (Drivers) and the inherent limitations in assay sensitivity and regulatory hurdles (Restraints). The primary driver is the sheer efficiency and cost-effectiveness of LFAs, making them ideal for high-volume screening and remote diagnostics, especially during pandemics or in low-resource settings. This momentum is further amplified by technological innovations aimed at improving quantitative analysis. However, a significant restraint remains the comparative lack of sensitivity and specificity of basic LFAs when benchmarked against gold-standard laboratory techniques like PCR or ELISA, leading to a risk of false negatives, which dampens their adoption for definitive diagnosis in some critical care scenarios. The overriding opportunity lies in expanding applications into non-traditional fields, such as veterinary diagnostics, environmental monitoring, and food safety testing, alongside developing advanced multiplex assays capable of detecting multiple targets simultaneously, thereby unlocking new revenue streams and market penetration.
Impact forces currently influencing the market include intense competition leading to pricing pressures, especially in high-volume, commodity-type assays (like certain pregnancy tests or basic drug screens). Regulatory compliance is a constantly evolving impact force; stringent standards in key markets like the FDA and CE-IVDR regions necessitate rigorous clinical validation, impacting time-to-market for new products. Furthermore, the competitive intensity is driving substantial investment in R&D focused on proprietary material science—specifically, novel conjugating agents and improved membrane matrices—to enhance flow characteristics and signal amplification. This technological arms race forces continuous product iteration among key players to maintain market relevance and secure competitive advantages in accuracy and speed, directly influencing purchasing decisions by large institutional buyers and public health agencies.
The convergence of digital health platforms and LFA technology represents a powerful impact force accelerating growth, transforming the LFA from a simple diagnostic strip into a connected data-generating device. This convergence addresses the historical limitation of LFAs being purely qualitative, enabling automated, quantitative data capture and integration into telemedicine workflows. However, maintaining supply chain resilience—ensuring the stable availability of critical components like nitrocellulose membranes and conjugated gold nanoparticles—remains a persistent logistical challenge, particularly given recent global supply chain disruptions. Successfully navigating these supply complexities and capitalizing on the shift toward digital integration are critical determinants of market success and sustained sales performance over the forecast period.
Segmentation Analysis
The Lateral Flow Assay (LFA) Sales Market is meticulously segmented based on product type, application, technique, and end-user, reflecting the diverse utility and technological variations within this rapid diagnostic platform. Understanding these segments is crucial for strategic market positioning and targeting specific buyer needs, ranging from large centralized laboratories seeking high-throughput readers to individual consumers requiring simple, reliable home testing kits. The segmentation highlights the underlying drivers of demand, where the volume of sales is often dictated by application (e.g., infectious diseases), while revenue generation is significantly influenced by the sophistication of the product (e.g., quantitative readers versus basic strips).
The core segmentation structure illustrates the market's maturity, with Kits & Reagents dominating in terms of volume due to their consumable nature, while the increasing adoption of immunochromatographic assays underscores the technical backbone of the industry. The highest growth potential lies within the quantitative and semi-quantitative segments, driven by professional end-users demanding clinical precision. Furthermore, the end-user segmentation shows a pivotal shift from professional care settings towards decentralized and consumer-centric points of care, necessitating specialized product designs that prioritize ease-of-use and stability outside controlled laboratory environments, thereby impacting distribution and sales strategies across all regions.
- Product Type:
- Kits & Reagents (Assay Strips, Membranes, Conjugates, Buffers)
- Readers (Digital, Optical, Fluorometric)
- Technique:
- Sandwich Assay
- Competitive Assay
- Multiplex Assay
- Application:
- Infectious Disease Testing (Respiratory, HIV, Hepatitis, Tropical Diseases)
- Pregnancy and Fertility Testing
- Cardiac Marker Testing
- Drugs of Abuse Testing
- Veterinary Diagnostics
- Food Safety & Environmental Testing
- End-User:
- Hospitals and Clinics
- Diagnostic Laboratories
- Home Care/Self-Testing Settings
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
Value Chain Analysis For Lateral Flow Assay Sales Market
The value chain for the Lateral Flow Assay Sales Market is characterized by a complex process starting from highly specialized upstream material suppliers and culminating in diverse downstream distribution channels reaching global end-users. Upstream analysis focuses on the sourcing and manufacturing of critical components, including high-quality nitrocellulose membranes, specific binding reagents (antibodies and antigens), and detection reagents (such as gold nanoparticles or fluorescent labels). Suppliers in this tier exert considerable influence over assay cost, quality, and stability, with strong negotiation power due to the specialized nature of these materials. Efficiency and reliability at this stage are paramount, as even minor variations in membrane porosity or conjugate consistency can critically impact the diagnostic performance of the final product. Vertical integration into membrane manufacturing is a growing trend among leading LFA developers to secure supply and maintain quality control.
The core manufacturing stage involves the dispensing, drying, assembly (lamination), and cutting of the components into finished test strips or cartridges. This stage requires rigorous quality assurance protocols and often involves proprietary automation technology to ensure batch-to-batch consistency—a critical factor for regulatory approval and market trust. Moving downstream, the distribution channel is highly fragmented and depends heavily on the target application and end-user. Direct sales are common for high-value contracts with large central laboratories or government health bodies, where technical support and integrated reader systems are key requirements. Conversely, indirect distribution utilizes a dense network of specialized medical distributors, wholesalers, and increasingly, e-commerce platforms, particularly for over-the-counter (OTC) home-use kits, necessitating efficient logistics and inventory management.
Direct channels offer better control over pricing and customer relationships, often employed for complex, professional-use diagnostic platforms where training and installation are required. Indirect channels, while adding layers of cost, provide essential market penetration into geographically dispersed or hard-to-reach healthcare facilities and retail pharmacies. The increasing adoption of decentralized testing has elevated the importance of pharmacy distribution and direct-to-consumer (D2C) online models for self-testing kits, requiring manufacturers to tailor packaging and instructions for non-professional use. Ensuring adequate shelf-life and robust packaging across these diverse channels is a crucial element of the downstream logistics strategy, directly influencing final sales figures and market accessibility.
Lateral Flow Assay Sales Market Potential Customers
Potential customers in the Lateral Flow Assay Sales Market represent a broad spectrum of healthcare providers, consumers, governmental agencies, and commercial entities, all seeking rapid, reliable, and portable diagnostic capabilities. The largest institutional buyers are hospitals and integrated healthcare networks (IHNs). These facilities procure high volumes of LFAs for immediate patient screening in emergency departments, decentralized hospital wards, and outpatient clinics, focusing on infectious disease panels and critical markers, thereby minimizing turnaround time for initial diagnosis before definitive lab tests are conducted. Their purchasing decisions are highly influenced by regulatory approval, clinical sensitivity data, and integration capabilities with existing hospital information systems (HIS).
Diagnostic laboratories, both large central labs and smaller satellite facilities, serve as crucial customers, particularly those adopting quantitative LFA readers. While they primarily handle high-complexity testing, LFAs are utilized for rapid preliminary screening, complementing their workflow by swiftly triaging samples. Moreover, governmental public health agencies and non-governmental organizations (NGOs) are significant purchasers, especially in large-scale procurement efforts for managing public health crises, vaccinations, and disease surveillance programs in low- and middle-income countries, where price sensitivity and ease of deployment are critical selection criteria for mass procurement contracts.
A rapidly expanding customer base includes individual consumers and home-users who drive the demand for over-the-counter (OTC) testing kits. This segment encompasses fertility monitoring, general wellness screening, and self-testing for common infectious agents, representing a key area for high-volume sales through retail pharmacies and e-commerce platforms. Additionally, customers extend beyond human health to include agricultural and food processing industries (purchasing assays for pathogen detection and quality control) and veterinary clinics (using LFAs for pet and livestock disease screening), demonstrating the cross-industry utility and expanding customer landscape of LFA technology.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 10.5 Billion |
| Market Forecast in 2033 | USD 18.8 Billion |
| Growth Rate | 8.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Abbott Laboratories, Danaher Corporation (Cepheid), F. Hoffmann-La Roche Ltd., Siemens Healthineers AG, QuidelOrtho Corporation, Becton, Dickinson and Company (BD), Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., BioMérieux SA, Meridian Bioscience, Inc., PerkinElmer Inc., EKF Diagnostics Holdings plc, Luminex Corporation (A DiaSorin Company), GenBody Inc., Guangzhou Wondfo Biotech Co., Ltd., Sekisui Diagnostics, Trinity Biotech plc, Access Bio Inc., Chembio Diagnostics, Inc., InBios International, Inc. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Lateral Flow Assay Sales Market Key Technology Landscape
The Lateral Flow Assay (LFA) technology landscape is rapidly evolving, moving beyond basic colloidal gold visualization to incorporate advanced materials and signal amplification techniques aimed at improving both sensitivity and quantitative measurement. A critical technological shift involves the transition from traditional visual interpretation to sophisticated digital reader systems. These readers utilize optical detection, fluorometry, or up-converting phosphor technology (UCP-LFA) to enhance the signal-to-noise ratio significantly, allowing for the detection of analytes at much lower concentrations, thereby bridging the performance gap with laboratory-based immunoassays. Furthermore, the integration of smartphone-based readers and cloud connectivity is streamlining data management, enabling instantaneous result logging, and facilitating integration into telemedicine workflows, which is paramount for decentralized care models.
Another major area of technological innovation is in the component materials themselves. Manufacturers are increasingly utilizing alternative nanoparticle labels, such as carbon nanoparticles, quantum dots, and magnetic beads, which offer superior signal intensity and stability compared to standard gold nanoparticles, particularly crucial for highly sensitive applications like cancer biomarker detection. Additionally, advancements in membrane engineering, including the development of tailored porous structures and surface chemistries, are optimizing fluid flow kinetics and maximizing the binding capacity of capture antibodies, consequently reducing non-specific binding and improving overall assay specificity. These material innovations are vital for the successful development of high-performance multiplex LFAs that can simultaneously detect several targets from a single sample volume, vastly increasing the diagnostic utility.
The future technology landscape is heavily invested in developing fully integrated, closed LFA cartridges that automate the sample preparation steps, mitigating potential user errors and standardizing the testing process. Microfluidic technologies are being combined with LFA formats to achieve precise control over sample movement and reaction timing, mimicking the precision of laboratory instruments in a disposable format. This convergence of microfluidics and LFAs is paving the way for next-generation POCT devices capable of performing complex diagnostic panels in non-laboratory settings. Furthermore, ongoing research focuses on embedding quality control indicators and incorporating anti-counterfeiting measures directly into the assay structure, ensuring the reliability and authenticity of diagnostic results across the global supply chain.
Regional Highlights
- North America: North America holds the largest revenue share in the LFA market, characterized by high healthcare expenditure, established government frameworks promoting POCT adoption, and substantial investments in R&D for advanced diagnostics. The region benefits from early market entry of novel technologies, particularly quantitative readers and sophisticated multiplex assays, driven by key market players headquartered here and strong demand from clinical laboratories and rapid emergency testing centers. High disease screening rates and a culture of proactive health management further cement its market leadership, although it is approaching maturity in terms of growth rate compared to Asia Pacific.
- Europe: Europe represents a mature market with robust growth, propelled by favorable regulatory environment (CE IVDR compliance driving quality improvements), strong public health systems prioritizing infectious disease control, and significant uptake of self-testing products. Western European countries like Germany, the UK, and France are the major revenue contributors. The market here is focused on optimizing cost-efficiency and integrating LFA results into centralized patient care records, often through government tenders and regional contracts with diagnostic providers, ensuring consistent demand for high-quality, regulated kits.
- Asia Pacific (APAC): APAC is anticipated to be the fastest-growing region during the forecast period due to burgeoning population density, improving healthcare infrastructure investments in emerging economies (India, China), and high prevalence of infectious diseases requiring mass screening programs. Government focus on establishing affordable and accessible diagnostic services, combined with increasing awareness and disposable incomes, is fueling massive unit volume sales. Local manufacturing and rapid scaling of production capabilities are key regional strategies, aiming to serve both domestic demand and export markets with cost-competitive LFA products.
- Latin America (LATAM): The LATAM market growth is driven by increasing public and private investments in healthcare infrastructure and efforts to manage endemic diseases like Dengue and Zika. The inherent portability and low cost of LFAs make them an ideal diagnostic solution for rural and remote areas where centralized laboratories are scarce. Market expansion is dependent on improving logistics chains and navigating diverse national regulatory pathways, with Brazil and Mexico leading the regional demand for both professional and consumer-grade testing kits.
- Middle East and Africa (MEA): MEA exhibits strong potential, particularly in addressing high burdens of infectious diseases such as malaria, HIV, and tuberculosis. Growth is largely supported by international funding initiatives (e.g., WHO, Global Fund) focused on decentralized diagnostic access. The adoption rate is accelerating due to the clinical utility of LFAs in areas with limited infrastructure. Key strategies involve direct engagement with government health ministries for mass procurement and establishing regional assembly plants to ensure timely supply and accessibility of critical rapid tests.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Lateral Flow Assay Sales Market.- Abbott Laboratories
- Danaher Corporation (Cepheid)
- F. Hoffmann-La Roche Ltd.
- Siemens Healthineers AG
- QuidelOrtho Corporation
- Becton, Dickinson and Company (BD)
- Thermo Fisher Scientific Inc.
- Bio-Rad Laboratories, Inc.
- BioMérieux SA
- Meridian Bioscience, Inc.
- PerkinElmer Inc.
- EKF Diagnostics Holdings plc
- Luminex Corporation (A DiaSorin Company)
- GenBody Inc.
- Guangzhou Wondfo Biotech Co., Ltd.
- Sekisui Diagnostics
- Trinity Biotech plc
- Access Bio Inc.
- Chembio Diagnostics, Inc.
- InBios International, Inc.
- ACON Laboratories, Inc.
- DRG International, Inc.
- Surmodics, Inc.
- Lohmann & Rauscher GmbH & Co. KG
- MilliporeSigma (Merck KGaA)
Frequently Asked Questions
Analyze common user questions about the Lateral Flow Assay Sales market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the projected Compound Annual Growth Rate (CAGR) for the Lateral Flow Assay Market through 2033?
The Lateral Flow Assay Sales Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.5% between 2026 and 2033, driven by increasing adoption of point-of-care testing and technological advancements in reader systems.
How do Lateral Flow Assays compare in sensitivity to traditional laboratory diagnostic methods like PCR or ELISA?
While LFAs offer superior speed and convenience, they generally possess lower sensitivity and specificity compared to laboratory-based methods such as Polymerase Chain Reaction (PCR) or Enzyme-Linked Immunosorbent Assay (ELISA). However, the performance gap is continually narrowing with the introduction of quantitative digital readers and advanced signal amplification techniques.
Which application segment drives the highest demand and sales volume in the LFA market?
The Infectious Disease Testing segment currently drives the highest demand and sales volume globally, particularly assays targeting respiratory pathogens (like influenza and COVID-19), due to widespread government procurement, routine screening requirements, and the necessity for rapid triage in clinical settings worldwide.
What is the major technological trend enhancing the capabilities of Lateral Flow Assays?
The major technological trend is the integration of digital reader systems, often utilizing fluorometric or advanced optical detection, which transforms qualitative results into quantitative or semi-quantitative data. This digitalization improves objectivity, reduces human error, and facilitates seamless data integration into health information systems (HIS).
Which region is expected to exhibit the fastest growth rate in the Lateral Flow Assay Sales Market?
The Asia Pacific (APAC) region is forecasted to exhibit the fastest growth rate, propelled by expanding healthcare infrastructure, increasing governmental initiatives for accessible diagnostics, and high demand for cost-effective rapid testing solutions across densely populated emerging economies such as China and India.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
