Lentinan Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 437597 | Date : Dec, 2025 | Pages : 249 | Region : Global | Publisher : MRU
Lentinan Market Size
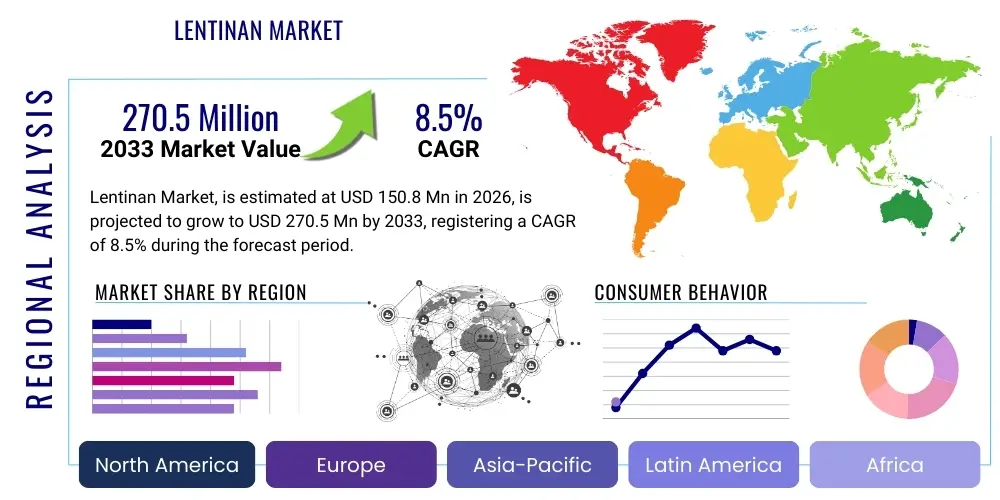
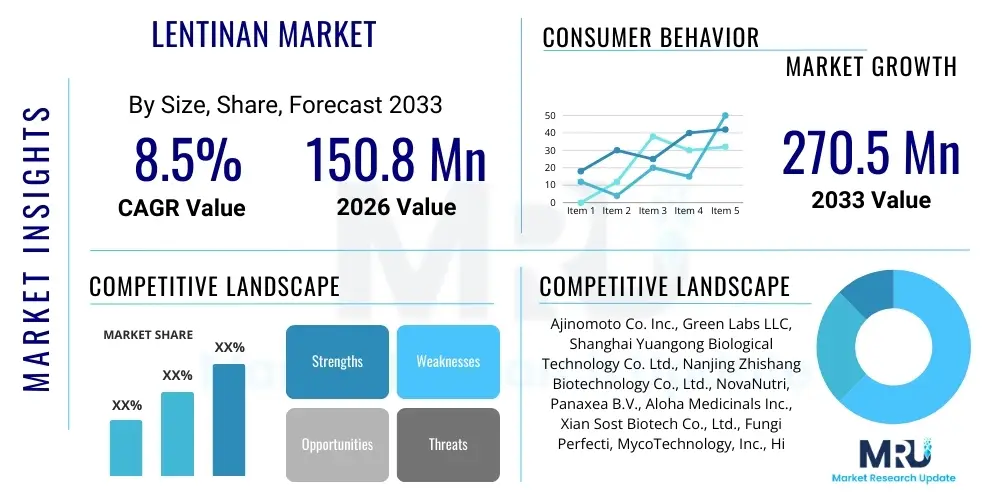
The Lentinan Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.5% between 2026 and 2033. The market is estimated at USD 150.8 Million in 2026 and is projected to reach USD 270.5 Million by the end of the forecast period in 2033.
Lentinan Market introduction
Lentinan is a highly purified polysaccharide derived primarily from the fruiting bodies of the Shiitake mushroom (Lentinus edodes). Chemically characterized as a β-(1,3)-(1,6)-glucan, it is renowned for its potent immunomodulatory and antitumor properties. Initially isolated and studied in Japan, lentinan has gained significant traction globally, transitioning from traditional medicine to a subject of rigorous clinical research, particularly as an adjuvant therapy in oncology. Its mechanism of action centers on stimulating host immune responses, including activating T-lymphocytes, Natural Killer (NK) cells, and macrophages, rather than directly targeting cancer cells, positioning it uniquely within the biological response modifier therapeutic class. The complex nature of its extraction and purification, essential for pharmaceutical-grade applications, contributes to its high value in the biomedical sector.
Major applications of Lentinan span Pharmaceuticals, where it is utilized as an injectable adjunct to chemotherapy and radiation therapy for various cancers, including gastric and colorectal cancer. Beyond oncology, its robust immunomodulating profile makes it valuable in Functional Foods and Nutraceuticals, aimed at general immune system support, and increasingly in specialized Cosmetics for skin health and anti-aging formulations. The driving factors for market expansion are multifaceted, including the global rise in cancer incidence, the accelerating consumer demand for natural, evidence-based immune-boosting supplements, and ongoing clinical trials validating its efficacy across diverse therapeutic areas. Furthermore, advancements in biotechnology, specifically in submerged fermentation techniques, are improving scalability and purity, mitigating historical reliance solely on traditional mushroom cultivation.
The product description highlights a shift towards high-purity, standardized extracts, essential for meeting stringent pharmaceutical regulatory requirements in North America and Europe. Key benefits include enhanced patient quality of life during conventional cancer treatments and low toxicity profiles compared to traditional chemotherapeutics. Market growth is further propelled by an aging global demographic, which necessitates advanced solutions for chronic disease management and proactive immune health maintenance. The market’s future trajectory is inextricably linked to continuous investment in sophisticated extraction technology and synthetic biology approaches designed to produce bio-identical or modified lentinan analogs efficiently and cost-effectively, ensuring a steady, high-quality supply chain to meet expanding global demand.
Lentinan Market Executive Summary
The Lentinan Market is witnessing robust growth, primarily driven by accelerated research in personalized oncology and the escalating consumer preference for high-quality functional ingredients globally. Key business trends indicate a strong move towards vertical integration among specialized biotech firms, aiming to control raw material sourcing—either through controlled mushroom cultivation or advanced microbial fermentation—to ensure purity and supply stability. The nutraceutical sector represents the fastest-growing end-use area, capitalizing on lentinan's immune-supportive claims, leading to increased product launches in dietary supplements, specialized beverages, and health-focused food items. Pricing volatility, historically linked to agricultural yields, is being stabilized by technological advancements, yet premium pricing remains characteristic for pharmaceutical-grade lentinan (>95% purity) due to intensive purification processes and regulatory validation costs.
Regional trends reveal Asia Pacific (APAC) maintaining its dominance, fueled by deeply entrenched traditional use of Shiitake mushrooms and the presence of major pharmaceutical manufacturers, especially in China and Japan, where lentinan is approved as an adjuvant cancer therapy. Conversely, North America and Europe are experiencing significant growth due to substantial investment in clinical trials and the increasing integration of integrative medicine practices, broadening physician acceptance of immunomodulatory polysaccharides. Regulatory harmonization, particularly concerning novel food status and therapeutic claims, remains a regional challenge, dictating the speed of market penetration outside established Asian markets. The focus on establishing clear bioavailability and safety data is paramount for further expansion in Western markets.
Segment trends underscore the segmentation by application, where Pharmaceuticals retain the largest revenue share, highly influenced by government healthcare spending and oncology treatment protocols. However, the Functional Foods and Beverages segment is poised for the highest Compound Annual Growth Rate, driven by innovative product formats like lentinan-infused gummies, sports nutrition powders, and specialized probiotic blends designed for gut-immune axis support. Within the source segment, synthetic or fermentation-derived lentinan is gaining prominence, promising greater batch consistency and eliminating the concerns associated with heavy metal contamination sometimes found in wild-harvested or traditionally cultivated sources. This shift towards synthetic biology methods is critical for meeting the scale and quality demands of global industrial customers.
AI Impact Analysis on Lentinan Market
Common user questions regarding the influence of Artificial Intelligence (AI) on the Lentinan Market frequently center on three critical areas: accelerating the drug discovery pipeline, optimizing bioprocessing efficiency, and personalizing treatment protocols. Users are keen to understand how computational models can identify novel therapeutic targets where lentinan or its derivatives might be effective, moving beyond established cancer applications. There is substantial interest in leveraging AI algorithms to analyze complex clinical data sets to predict patient responses to lentinan adjuvant therapy, thereby refining personalized medicine strategies and reducing the cost of ineffective treatments. Additionally, questions often arise about using machine learning to optimize fermentation parameters (e.g., nutrient levels, temperature, pH) in real-time, drastically improving lentinan yield and purity while lowering production costs, thus addressing a major market restraint.
The collective expectations surrounding AI suggest a transformative impact on the market's efficiency and expansion capabilities. AI tools are expected to play a crucial role in managing the complex data generated during the purification and quality control phases, ensuring that batches meet ultra-high pharmaceutical standards consistently. Specifically, deep learning models can analyze chromatographic data and spectroscopic signatures to rapidly detect impurities or variations in polysaccharide structure that manual analysis might miss, enhancing product safety. Furthermore, predictive modeling powered by AI assists in understanding the complex interactions between lentinan and the human immune system at a cellular level, aiding researchers in developing more potent and stable delivery systems, such as targeted liposomes or nanoparticles designed to maximize bioavailability in specific anatomical sites.
- AI-driven optimization of submerged fermentation bioreactors to maximize lentinan yield and structural integrity.
- Machine learning for real-time quality assurance and contamination detection in high-purity extracts.
- Computational modeling to predict the efficacy of lentinan as an adjuvant therapy based on genetic and immunological patient data.
- AI acceleration of clinical trial analysis and patient recruitment for novel applications of lentinan.
- Development of novel polysaccharide derivatives through AI-guided synthetic modification to enhance stability and targeting specificity.
DRO & Impact Forces Of Lentinan Market
The dynamics of the Lentinan Market are significantly shaped by a confluence of accelerating drivers and constraining factors, balanced by emerging opportunities. The primary driver is the scientifically validated evidence of lentinan's role as a biological response modifier, particularly its successful application as an adjuvant in oncology treatment protocols in established Asian markets. This efficacy, combined with the rising global expenditure on complementary and alternative medicine (CAM) and a palpable consumer desire for natural, immune-boosting ingredients, provides sustained momentum. However, restraints include the high production cost associated with achieving pharmaceutical-grade purity, the vulnerability of agricultural sourcing (Shiitake cultivation) to environmental factors, and complex regulatory pathways in Western jurisdictions that demand extensive, costly clinical trials for new therapeutic claims, particularly in injectable forms. These constraints necessitate substantial upfront capital investment.
Opportunities for growth are concentrated in two primary domains: technological innovation and therapeutic diversification. The advent of synthetic biology and advanced microbial fermentation offers a scalable, controlled, and consistent method for high-volume lentinan production, mitigating agricultural supply chain risks and improving cost efficiency. Simultaneously, expansion into non-oncology applications—such as chronic inflammatory diseases, antiviral research, and veterinary immunotherapeutics—presents significant untapped revenue streams. Developing novel delivery technologies, including encapsulation and nanoparticle carriers, represents a critical opportunity to enhance oral bioavailability, which is a key barrier to widespread adoption outside hospital settings, thus expanding its reach into the consumer nutraceutical space.
Impact forces exert constant pressure on market stakeholders. Supplier concentration remains moderate, primarily centered around Asian producers, giving them leverage over upstream pricing, yet intense competition in the downstream functional food segment keeps consumer prices elastic. Governmental health policies, particularly national guidelines concerning the reimbursement of adjuvant therapies and the approval processes for biological response modifiers, directly dictate the growth trajectory of the pharmaceutical segment. The threat of substitution, mainly from other medicinal mushroom extracts (e.g., Reishi, Cordyceps) or synthetic immunomodulators, necessitates continuous R&D investment to maintain lentinan's competitive edge and validate its superior structural and functional efficacy. Regulatory barriers demanding extensive safety and standardization data represent a high entry barrier for new competitors seeking to enter the high-purity market.
Segmentation Analysis
The Lentinan market is comprehensively segmented based on its source material, application area, required purity level, and final form, reflecting the diverse end-user requirements from highly regulated pharmaceutical use to mass-market functional foods. Analysis of these segments is crucial for understanding specific market dynamics, pricing strategies, and regional variances in demand. The source segmentation highlights the ongoing transition from traditional Shiitake extraction to more technologically advanced fermentation methods, driven by industrial needs for consistency. Application analysis clearly defines revenue streams, with the established medical segment supporting high profitability and the burgeoning nutraceutical sector driving volume growth. Purity level segmentation is essential as it dictates the suitability for injectable applications versus oral supplements, directly impacting manufacturing complexity and final product cost.
- Source
- Shiitake Mushroom Extract (Traditional)
- Fermentation/Synthetic (Microbial)
- Application
- Pharmaceuticals (Oncology, Immunotherapy)
- Functional Foods and Beverages
- Dietary Supplements (Nutraceuticals)
- Cosmetics and Personal Care
- Animal Feed and Veterinary Medicine
- Purity Level
- Low Purity (50%-70%)
- Medium Purity (70%-90%)
- High Purity (>90%)
- Form
- Powder
- Liquid/Injectable Solutions
Value Chain Analysis For Lentinan Market
The Lentinan value chain begins with rigorous upstream activities focused on raw material production, primarily through the cultivation of Lentinus edodes (Shiitake) mushrooms or through specialized microbial fermentation processes. Upstream analysis highlights the critical importance of selecting high-yield fungal strains and maintaining stringent environmental controls (temperature, humidity, substrate composition) to ensure optimal polysaccharide content. For fermentation-derived lentinan, the upstream phase involves designing and scaling up bioreactor processes, nutrient media optimization, and genetic stability monitoring of the microbial strains. Quality control at this initial stage is paramount, as the presence of heavy metals or agricultural contaminants severely compromises suitability for pharmaceutical applications, necessitating careful sourcing and validation of the raw materials before extraction commences.
The midstream phase involves complex and capital-intensive purification and processing activities. Extraction methods, such as hot water extraction followed by alcohol precipitation, are critical for isolating the crude lentinan. Subsequent refinement to achieve pharmaceutical-grade purity involves advanced chromatography and filtration techniques, significantly adding to the production cost. The finished product then enters the downstream distribution channel. Direct distribution, involving specialized logistics for temperature-sensitive injectable forms, is characteristic of the pharmaceutical segment, often dealing directly with hospitals, clinics, and compounding pharmacies. This channel requires rigorous adherence to Good Manufacturing Practices (GMP) and detailed traceability protocols, ensuring patient safety and regulatory compliance across international borders.
Conversely, indirect distribution channels dominate the nutraceutical and functional food segments. Here, lentinan powder is sold in bulk or semi-processed forms to ingredient distributors, contract manufacturers, and supplement brands. These entities then incorporate the lentinan into consumer-facing products (capsules, tablets, functional drinks) and utilize extensive retail networks, including e-commerce platforms, health food stores, and pharmacies. The efficiency of the indirect channel hinges on effective marketing and robust labeling to communicate product efficacy to the end consumer. The value addition throughout the chain is highest in the purification stage, followed by brand marketing and final formulation, distinguishing generic low-purity ingredients from high-value, standardized therapeutic products.
Lentinan Market Potential Customers
The primary consumers and beneficiaries of the Lentinan Market are highly specialized and diversified across various industries. Pharmaceutical and biopharmaceutical companies constitute the most significant customer segment, particularly those focused on oncology, immunology, and adjuvant therapies, purchasing high-purity lentinan for clinical formulation and subsequent injection into cancer patients as part of conventional treatment protocols. These buyers prioritize purity, consistency, regulatory documentation (DMF filings), and supplier accreditation (e.g., cGMP compliance) above all else, often engaging in long-term supply agreements to secure consistent quality batches for their ongoing drug development and commercial production cycles.
A rapidly expanding customer base includes Nutraceutical and Dietary Supplement manufacturers globally. These companies target health-conscious consumers seeking natural ingredients for preventative health, immune system boosting, and vitality enhancement. They generally utilize medium to low-purity lentinan powder in capsules, blends, and functional food matrices. Furthermore, specialty manufacturers in the Cosmeceutical sector are emerging customers, incorporating lentinan into anti-aging creams and skin treatments due to its purported antioxidant and immune-modulating effects on skin health. Lastly, the Animal Health sector represents a niche but growing customer segment, utilizing lentinan in specialized feed additives to enhance the immune response and general health of livestock and companion animals, thereby reducing reliance on antibiotics.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 150.8 Million |
| Market Forecast in 2033 | USD 270.5 Million |
| Growth Rate | 8.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Ajinomoto Co. Inc., Green Labs LLC, Shanghai Yuangong Biological Technology Co. Ltd., Nanjing Zhishang Biotechnology Co., Ltd., NovaNutri, Panaxea B.V., Aloha Medicinals Inc., Xian Sost Biotech Co., Ltd., Fungi Perfecti, MycoTechnology, Inc., Hi-Tech Pharmaceuticals, Inc., Nammex (North American Medicinal Mushroom Extracts), Sunchang Industrial Development Co., Ltd., Xi'an Herbking Biotechnology Co., Ltd., Hubei Haoyuan Biotechnology Co., Ltd., Bio-Active Co. Ltd., Hebei Cangyanshan Food Co. Ltd., Zhejiang Shouxiangu Botanical Technology Co., Ltd., Kikkoman Corporation, TCI Chemicals. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Lentinan Market Key Technology Landscape
The technological landscape driving the Lentinan market is bifurcated between optimizing traditional extraction methods and pioneering advanced biological synthesis techniques. In extraction, the focus is on enhancing yield and maintaining structural integrity. Modern technologies such as Ultrasonic-Assisted Extraction (UAE) and Microwave-Assisted Extraction (MAE) are increasingly replacing conventional hot water extraction, offering faster processing times, lower energy consumption, and superior extraction efficiency. Furthermore, Supercritical Fluid Extraction (SFE), particularly using CO2, is being explored for the final purification steps, enabling solvent-free and residue-free isolation of high-purity lentinan suitable for injectable pharmaceutical applications, a crucial factor for gaining regulatory approval in stringent Western markets.
The most transformative technological shift involves microbial fermentation, often referred to as synthetic biology or biosynthesis. This technique utilizes submerged cultivation in large bioreactors, offering complete control over the growth environment for selected fungal or yeast strains that produce bio-identical lentinan. This approach addresses the inherent limitations of traditional Shiitake cultivation, such as seasonal variability, land use requirements, and inconsistency in polysaccharide yields. Fermentation technology ensures scalable, continuous, and high-purity production, enabling manufacturers to meet the burgeoning industrial demand from the functional food sector and reducing long-term dependence on agricultural commodities. Furthermore, R&D is heavily focused on enzymatic modification technologies, aiming to produce chemically modified lentinan derivatives with enhanced stability, solubility, and targeted pharmacological activities.
Technological advancement is also pivotal in quality control and standardization. High-Performance Liquid Chromatography (HPLC), Gel Permeation Chromatography (GPC), and advanced Nuclear Magnetic Resonance (NMR) spectroscopy are standard techniques utilized to precisely characterize the molecular weight and structure of the polysaccharide, which is directly correlated with its immunological activity. Maintaining precise consistency in these complex biological macromolecules is a major technical challenge, and the deployment of advanced analytical robotics and AI-integrated quality management systems is becoming standard practice among leading manufacturers to comply with global pharmaceutical standards and secure long-term contracts in the high-value therapeutic segment.
Regional Highlights
- Asia Pacific (APAC): APAC is the largest market segment for lentinan, dominating both consumption and production. This supremacy stems from the historical and cultural acceptance of Shiitake mushrooms and lentinan's established status as an approved adjunct cancer treatment in countries like Japan and China. Japan, in particular, was instrumental in lentinan's early development and clinical integration. China remains the largest producer of raw materials and primary extracts, benefiting from low-cost labor and extensive cultivation expertise. Market growth in Southeast Asia is driven by the rapid expansion of the dietary supplement industry and increasing consumer wealth allowing for higher expenditure on preventative health products. The region is characterized by high volume, varying purity standards, and increasingly sophisticated manufacturing infrastructure focusing on export-grade materials.
- North America (NA): North America represents a high-growth market, primarily driven by the increasing acceptance of integrative oncology practices and a robust nutraceutical industry. While formal pharmaceutical approval for lentinan as a standalone drug is less common than in Asia, its inclusion in high-end dietary supplements and physician-prescribed natural health products is accelerating. The market here demands superior transparency, stringent third-party testing, and non-GMO sourcing. Growth is highly influenced by consumer education regarding immune health benefits and rising healthcare costs prompting patients to seek complementary therapies.
- Europe: The European market is characterized by slow but steady growth, heavily influenced by varied national regulatory frameworks regarding food supplements and novel foods. Germany and the UK are key markets, showing high demand in the medical mushroom extract segment. Regulatory hurdles under the European Food Safety Authority (EFSA) for health claims and Novel Food status dictate market entry strategies. European manufacturers often focus on highly standardized, certified organic, or fermentation-derived products to meet sophisticated consumer demands and regulatory compliance, particularly for claims related to immune support and vitality.
- Latin America and Middle East & Africa (MEA): These regions currently represent smaller market shares but offer significant future potential. Growth in Latin America is tied to improving healthcare infrastructure and growing middle-class expenditure on health and wellness. In MEA, market penetration is slower due to fragmented distribution channels and lower awareness, but increasing cancer incidence rates and rising medical tourism present future opportunities, especially for imported, high-quality pharmaceutical lentinan from established Asian and European suppliers.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Lentinan Market.- Ajinomoto Co. Inc.
- Green Labs LLC
- Shanghai Yuangong Biological Technology Co. Ltd.
- Nanjing Zhishang Biotechnology Co., Ltd.
- NovaNutri
- Panaxea B.V.
- Aloha Medicinals Inc.
- Xian Sost Biotech Co., Ltd.
- Fungi Perfecti
- MycoTechnology, Inc.
- Hi-Tech Pharmaceuticals, Inc.
- Nammex (North American Medicinal Mushroom Extracts)
- Sunchang Industrial Development Co., Ltd.
- Xi'an Herbking Biotechnology Co., Ltd.
- Hubei Haoyuan Biotechnology Co., Ltd.
- Bio-Active Co. Ltd.
- Hebei Cangyanshan Food Co. Ltd.
- Zhejiang Shouxiangu Botanical Technology Co., Ltd.
- Kikkoman Corporation
- TCI Chemicals
Frequently Asked Questions
Analyze common user questions about the Lentinan market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary mechanism of action of lentinan and why is it used in oncology?
Lentinan is classified as a Biological Response Modifier (BRM). Its primary mechanism is not direct toxicity to cancer cells but the potent stimulation of the host immune system. It specifically activates macrophages, T-helper cells, and Natural Killer (NK) cells, enhancing the body’s ability to detect and fight tumors, making it effective as an adjuvant therapy alongside chemotherapy or radiation to improve patient outcomes and quality of life.
What are the key technological advancements driving production efficiency in the Lentinan Market?
The market is increasingly relying on advanced technologies such as microbial submerged fermentation, which provides a controllable, scalable, and consistent method for high-purity lentinan synthesis, overcoming the limitations and variability associated with traditional Shiitake mushroom cultivation. Furthermore, advanced purification techniques like Supercritical Fluid Extraction are crucial for achieving pharmaceutical-grade standards.
How does the purity level of lentinan affect its market application and pricing?
Purity level directly determines the application and price. High-purity lentinan (typically >90%) is required for injectable pharmaceutical applications due to stringent safety and regulatory standards, commanding the highest premium. Lower purity grades are generally utilized in high-volume, lower-cost consumer segments such as nutraceuticals, functional foods, and animal feed.
Which regional market holds the greatest potential for future growth outside of Asia Pacific?
North America is projected to exhibit the fastest growth outside APAC. This expansion is fueled by rising consumer interest in immunomodulatory supplements, increased acceptance of integrative medicine by healthcare professionals, and significant R&D investments aimed at clinical validation and enhanced delivery system development, particularly within the dietary supplement and specialty health product sectors.
What major regulatory challenges currently face lentinan manufacturers seeking global market entry?
Major challenges include navigating fragmented regulatory frameworks regarding classification, especially in Europe (Novel Food status) and North America (GRAS status). Furthermore, manufacturers seeking therapeutic claims must undertake extensive and expensive clinical trials to demonstrate efficacy and safety, particularly for injectable formulations, and must strictly adhere to international cGMP standards to ensure product consistency.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
- Lentinan Market Size Report By Type (Food Grade, Pharmaceutical Grade, Other), By Application (Food additive, Health product field, Anti-cancer drug, Others), By Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Share, Trends, Outlook and Forecast 2025-2032
- Shiitake Mushroom Extract Market Statistics 2025 Analysis By Application (Functional Food, Health Care Products, Cosmetics, The Pharmaceutical), By Type (Lentinan 20%, Lentinan 30%, Lentinan 50%, Others), and By Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Forecast 2025 to 2032
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager