Mechanical Chest Compression Devices Market Size, By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 439765 | Date : Jan, 2026 | Pages : 242 | Region : Global | Publisher : MRU
Mechanical Chest Compression Devices Market Size
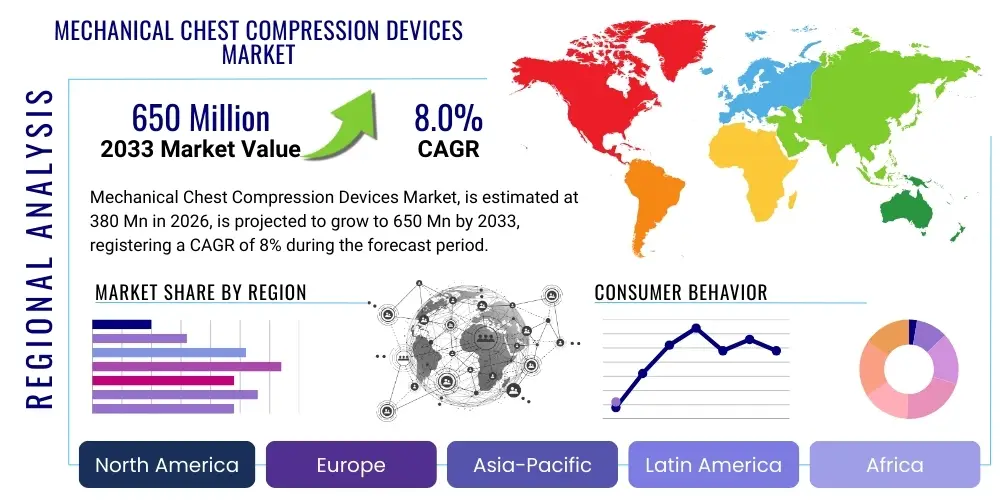
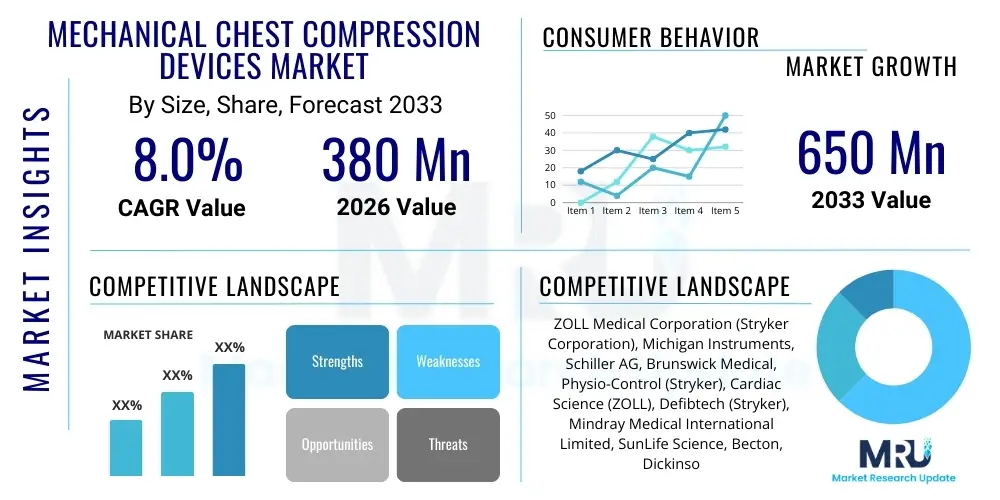
The Mechanical Chest Compression Devices Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.0% between 2026 and 2033. The market is estimated at 380 Million USD in 2026 and is projected to reach 650 Million USD by the end of the forecast period in 2033.
Mechanical Chest Compression Devices Market introduction
The Mechanical Chest Compression Devices Market encompasses a range of automated medical devices designed to deliver high-quality, consistent chest compressions during cardiopulmonary resuscitation (CPR). These devices are engineered to overcome the inherent challenges of manual CPR, such as rescuer fatigue, inconsistent compression depth and rate, and the difficulty of performing CPR in moving environments like ambulances or during patient transport. By automating the compression process, mechanical devices aim to ensure optimal blood flow to vital organs, significantly enhancing the chances of survival and improving neurological outcomes for patients experiencing sudden cardiac arrest. The introduction of these devices has marked a significant advancement in emergency medical care, providing a standardized approach to a critical life-saving intervention.
The primary product offerings in this market include piston-based devices and load-distributing band devices, each employing different mechanisms to achieve rhythmic chest compressions. Piston-based systems often utilize a plunger mechanism directly over the sternum, while load-distributing band devices wrap around the patient's chest, applying pressure circumferentially. Major applications for these devices span pre-hospital emergency medical services, in-hospital critical care units, catheterization laboratories, and inter-hospital patient transport. The versatility and adaptability of these devices to various clinical settings underscore their importance in modern resuscitation protocols. They serve as a critical tool for healthcare providers, offering a reliable alternative when manual CPR is impractical or less effective.
The benefits derived from mechanical chest compression devices are multifaceted, extending from enhanced patient care to improved rescuer safety and operational efficiency. For patients, the primary advantage lies in the consistent delivery of compressions, minimizing interruptions and maintaining crucial blood flow. For rescuers, these devices reduce physical strain and fatigue, allowing them to focus on other critical aspects of patient care, especially during prolonged resuscitation efforts or in challenging environments. The driving factors behind the market's growth include the increasing incidence of sudden cardiac arrest globally, a rising awareness among healthcare professionals regarding the benefits of consistent CPR, stringent guidelines from resuscitation councils advocating for high-quality CPR, and continuous technological advancements aimed at improving device portability, user-friendliness, and integration with advanced life support systems.
Mechanical Chest Compression Devices Market Executive Summary
The Mechanical Chest Compression Devices Market is undergoing a transformative period, characterized by robust business trends focusing on innovation, integration, and strategic partnerships. Manufacturers are increasingly investing in research and development to enhance device portability, battery life, and overall ease of use, making these life-saving tools more accessible and effective in diverse clinical and pre-hospital environments. A notable business trend involves the consolidation of market players through mergers and acquisitions, aiming to expand product portfolios and geographical reach, thereby streamlining market competition and fostering comprehensive solutions. Furthermore, there is a growing emphasis on data connectivity and intelligent feedback systems that can provide real-time performance metrics, which helps improve the quality of resuscitation efforts and facilitates post-event analysis for continuous improvement in patient care protocols.
Regional trends significantly influence the adoption and growth trajectory of mechanical chest compression devices. North America and Europe currently dominate the market, primarily due to well-established emergency medical services infrastructures, high awareness levels among medical professionals, and favorable reimbursement policies. These regions are also at the forefront of technological adoption and clinical research supporting the efficacy of these devices. However, the Asia Pacific region is rapidly emerging as a high-growth market, driven by improving healthcare infrastructure, increasing prevalence of cardiovascular diseases, rising disposable incomes, and government initiatives aimed at modernizing emergency medical care. Latin America, the Middle East, and Africa are also witnessing gradual market penetration, fueled by increasing investments in healthcare facilities and growing awareness, though challenges such as high initial costs and limited infrastructure persist in some areas.
Segmentation trends within the market highlight a shift towards more versatile and user-friendly devices. The demand for portable mechanical chest compression devices is steadily increasing, driven by their critical utility in pre-hospital settings, emergency transport, and disaster response scenarios where mobility is paramount. There is also a distinct segmentation in application, with significant demand from both in-hospital settings, such as emergency departments and cardiac catheterization labs, and pre-hospital environments including ambulances and air medical services. End-user segments, predominantly hospitals and emergency medical service providers, are seeking devices that offer seamless integration with existing resuscitation guidelines and training programs, emphasizing ease of deployment and minimal interruption to ongoing patient care. These segment-specific demands are prompting manufacturers to develop tailored solutions that cater to the unique operational requirements of different healthcare providers.
AI Impact Analysis on Mechanical Chest Compression Devices Market
User inquiries regarding the influence of Artificial Intelligence (AI) on Mechanical Chest Compression Devices frequently revolve around its potential to enhance CPR efficacy, provide real-time adaptive feedback, integrate with predictive analytics for cardiac arrest outcomes, and optimize device performance and maintenance. Common questions explore how AI could lead to more personalized resuscitation efforts, moving beyond standardized protocols to adaptive, patient-specific interventions. Users are also keen to understand AI's role in streamlining training processes, improving operational efficiency, and potentially reducing human error during critical moments. These themes underscore a collective expectation that AI will bring a new level of precision, intelligence, and predictive capability to mechanical chest compression, ultimately aiming for superior patient outcomes.
The integration of Artificial Intelligence into mechanical chest compression devices is poised to revolutionize cardiac arrest management by introducing intelligent automation and data-driven decision-making. AI algorithms can process vast amounts of real-time physiological data, including ECG readings, end-tidal CO2 levels, and blood pressure, to dynamically adjust compression parameters such as depth, rate, and recoil. This adaptive approach moves beyond fixed protocols, allowing devices to deliver CPR tailored to an individual patient's specific needs and response, optimizing blood flow and oxygen delivery to vital organs. Furthermore, AI can contribute significantly to predictive analytics, enabling earlier identification of patients at high risk of cardiac arrest and potentially guiding pre-emptive interventions or more rapid deployment of mechanical compression devices.
Beyond immediate patient care, AI also holds immense potential for operational improvements and training within the mechanical chest compression devices market. AI-powered systems can analyze performance data from countless resuscitation events, identifying patterns and areas for improvement in both device design and user technique. This data-driven insight can inform the development of next-generation devices that are more intuitive, robust, and effective. For training, AI can create highly realistic simulation environments, offering personalized feedback to healthcare professionals on their CPR skills, including the optimal application and monitoring of mechanical devices. Moreover, AI can enhance device maintenance by predicting potential failures or required servicing, ensuring devices are always in optimal working condition when needed most. The ethical implications of AI in life-or-death situations, data privacy, and regulatory approval will remain critical considerations as this technology evolves.
- Real-Time Adaptive Compression: AI algorithms analyze patient physiology (e.g., end-tidal CO2, blood pressure) to dynamically adjust compression depth, rate, and recoil for optimal blood flow, moving beyond fixed protocols to personalized CPR.
- Predictive Analytics for Outcomes: AI can process patient data to predict the likelihood of successful resuscitation or neurological outcomes, helping clinicians make more informed decisions about ongoing care and resource allocation.
- Enhanced Feedback and Training: AI-driven systems provide immediate, objective feedback on CPR quality during live events and simulations, enabling healthcare providers to refine their skills and optimize device usage.
- Integration with Advanced Life Support: AI facilitates seamless integration of mechanical compression devices with other monitoring equipment and electronic health records, creating a comprehensive data stream for holistic patient management.
- Automated Data Analysis and Reporting: AI can automatically log and analyze performance data from each resuscitation event, generating detailed reports for quality improvement, debriefing, and adherence to guidelines.
- Device Maintenance and Diagnostics: AI can monitor device health, predict potential mechanical failures, and recommend proactive maintenance, ensuring devices are always operational and reliable for critical use.
- Optimized Workflow and Deployment: AI could assist in determining optimal device placement and configuration based on patient anatomy and environmental factors, reducing setup time and improving efficacy.
- Research and Development Acceleration: AI can analyze large datasets from clinical trials and real-world usage to identify effective compression strategies and inform the design of future, more advanced mechanical devices.
DRO & Impact Forces Of Mechanical Chest Compression Devices Market
The Mechanical Chest Compression Devices Market is shaped by a complex interplay of various factors that collectively drive its growth, pose limitations, open new avenues for expansion, and exert overarching pressures. The primary drivers fueling this market include the escalating global incidence of sudden cardiac arrest, which necessitates effective and immediate interventions. Concurrently, increasing awareness among healthcare professionals and emergency responders about the limitations of manual CPR, particularly regarding consistency and rescuer fatigue during prolonged resuscitation, is prompting a shift towards automated solutions. Furthermore, evolving clinical guidelines from major resuscitation councils, such as the American Heart Association (AHA) and the European Resuscitation Council (ERC), increasingly emphasize high-quality, uninterrupted chest compressions, thereby advocating for the use of mechanical devices to achieve these standards. Technological advancements leading to more portable, user-friendly, and efficient devices also serve as a significant driver, enhancing their appeal and applicability in diverse emergency settings.
Despite the compelling drivers, several restraints challenge the widespread adoption of mechanical chest compression devices. The most significant impediment is the high initial cost of these advanced medical devices, which can be a substantial financial burden for healthcare facilities and emergency medical services, especially in developing regions with constrained budgets. This cost factor also impacts the availability of adequate devices across all necessary settings. Another restraint stems from the logistical complexities associated with device deployment, including the need for specialized training for operators, regular maintenance, and challenges in rapid application in chaotic emergency scenarios. Moreover, a degree of resistance to change from traditional manual CPR practices, along with concerns regarding potential patient injuries such as fractures or soft tissue damage, while generally low with proper use, can also slow adoption rates. Finally, the varied regulatory landscape across different countries adds complexity for manufacturers seeking to market their products globally, requiring adherence to diverse standards and approval processes.
Opportunities for growth and innovation within the Mechanical Chest Compression Devices Market are abundant, particularly in underserved geographical areas and through technological advancements. Emerging economies in Asia Pacific, Latin America, and the Middle East and Africa present significant untapped market potential as healthcare infrastructures improve and awareness regarding advanced resuscitation techniques grows. There is a strong opportunity for the integration of mechanical chest compression devices with broader advanced life support systems, creating more cohesive and data-rich resuscitation platforms. The ongoing development of more compact, lightweight, and versatile devices that can be deployed rapidly and efficiently in diverse environments, including austere conditions, offers another significant avenue for market expansion. Furthermore, opportunities exist in developing personalized CPR algorithms, potentially leveraging artificial intelligence, to optimize compressions based on individual patient characteristics and real-time physiological responses, thereby maximizing treatment efficacy and improving outcomes. Enhanced training programs and simulations to overcome operational barriers also represent a key opportunity.
Segmentation Analysis
The Mechanical Chest Compression Devices Market is broadly segmented to provide a comprehensive understanding of its various facets, allowing for targeted strategic planning and market penetration. These segmentations are critical for analyzing market dynamics, identifying specific growth areas, and understanding the diverse needs of end-users across different applications and geographical regions. The market can be dissected based on product type, portability, application, and end-user, each revealing unique trends and demand patterns. This structured approach to market analysis ensures that all critical dimensions are considered, offering a granular view of where growth is concentrated and how technological advancements are influencing specific sub-markets. Understanding these segments is paramount for manufacturers and service providers looking to innovate and expand their footprint effectively.
- By Product Type:
- Piston-Based Devices: These devices typically use a electrically powered pneumatic or electro-mechanical piston to apply direct, rhythmic compressions to the patient's sternum. They are highly effective in delivering consistent depth and rate, mimicking manual CPR but with superior consistency. Examples include the LUCAS device.
- Load-Distributing Band Devices: These systems employ a wide band that wraps around the patient's chest, distributing compression forces over a larger area. This design aims to provide circumferential chest compressions, which some studies suggest can improve blood flow. The AutoPulse system is a prominent example of this type.
- By Portability:
- Portable Devices: Designed for ease of transport and rapid deployment in various emergency settings, including ambulances, air medical services, and rugged outdoor environments. These devices are typically lighter, more compact, and battery-operated, making them ideal for pre-hospital care and inter-facility transfers.
- Stationary Devices: Primarily used in fixed healthcare settings such as hospital emergency departments, intensive care units, and catheterization laboratories. While still movable within a facility, their design prioritizes robust performance and integration with other hospital equipment over extreme portability.
- By Application:
- Pre-hospital Emergency Medical Services (EMS): This segment covers the use of devices by paramedics, EMTs, and other first responders at the scene of an emergency and during transport to a medical facility. The need for consistent compressions in moving vehicles makes mechanical devices highly valuable here.
- In-hospital Applications: Includes usage in emergency rooms, cardiac catheterization labs, operating rooms, and intensive care units where patients may experience in-hospital cardiac arrest. These settings benefit from the device's ability to maintain high-quality CPR during complex medical procedures or extended resuscitation efforts.
- Transport/Inter-facility Transfer: Involves the use of these devices to maintain CPR during the transfer of critically ill patients between different healthcare facilities or within a single large hospital complex.
- Military Medical Units: Deployment in field hospitals and tactical evacuation scenarios where consistent CPR is vital under challenging operational conditions.
- By End-User:
- Hospitals: The largest end-user segment, encompassing emergency departments, ICUs, CCUs, and cath labs. Hospitals require multiple devices to serve various departments and patient needs.
- Emergency Medical Services (EMS) Providers: Ambulance services, fire departments with medical response capabilities, and other pre-hospital care providers.
- Ambulatory Surgical Centers (ASCs) & Specialty Clinics: While less frequent, some ASCs or clinics performing procedures with cardiac risk may opt for these devices.
- Military & Defense Medical Facilities: For use in combat zones, military hospitals, and during medical evacuations.
- Training & Simulation Centers: Institutions that use these devices for advanced resuscitation training for medical professionals.
- By Region:
- North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA). Each region exhibits distinct market characteristics driven by healthcare infrastructure, regulatory environment, and economic development.
Value Chain Analysis For Mechanical Chest Compression Devices Market
The value chain for the Mechanical Chest Compression Devices Market is a sophisticated network encompassing various stages, from raw material sourcing and manufacturing to distribution and end-user engagement. At the upstream analysis stage, the market relies heavily on a specialized ecosystem of suppliers providing high-precision components and materials. This includes manufacturers of advanced electronic components such as microcontrollers, sensors for monitoring compression parameters, and robust battery technologies like lithium-ion for portable devices. Mechanical components such as precision motors, gearboxes, and durable plastics and metals for the device chassis and patient contact surfaces are also critical. The quality and reliability of these upstream components directly impact the performance, durability, and safety of the final product. Strong relationships with reliable and certified suppliers are essential to ensure a consistent supply chain, maintain quality control, and integrate the latest technological advancements into device manufacturing.
Moving through the value chain, the manufacturing phase involves intricate assembly, rigorous testing, and quality assurance processes. Companies invest significantly in research and development to innovate device design, improve user interfaces, and enhance overall efficacy in clinical settings. This stage also includes compliance with stringent medical device regulations from bodies like the FDA in the US, CE Mark in Europe, and similar authorities globally, which dictate design, manufacturing practices, and post-market surveillance. Once manufactured, the downstream analysis focuses on how these sophisticated devices reach the end-users. This involves a complex web of distribution channels, training, and ongoing support. The efficiency of the downstream segment is critical for market penetration and user satisfaction, as timely delivery, proper installation, and comprehensive training are vital for effective product utilization in emergency situations.
Distribution channels for mechanical chest compression devices are typically multifaceted, involving both direct and indirect approaches. Direct distribution channels are often employed by larger manufacturers who have established in-house sales teams and technical support personnel to directly engage with major institutional buyers such as large hospital networks, national EMS providers, and government agencies. This direct approach allows for closer client relationships, customized solutions, and direct feedback loops for product improvement. Indirect distribution, on the other hand, involves leveraging a network of third-party distributors, wholesalers, and medical device retailers. These partners often have established regional presence, local market knowledge, and existing relationships with smaller hospitals, private clinics, and individual EMS units, enabling broader market reach. Both direct and indirect channels often integrate comprehensive after-sales support, including maintenance, repairs, and refresher training, which are crucial for ensuring device longevity and optimal performance in critical care scenarios. The choice and balance between direct and indirect channels are often determined by geographical market specifics, target customer segments, and the manufacturer's overall business strategy.
Mechanical Chest Compression Devices Market Potential Customers
The primary potential customers and end-users for Mechanical Chest Compression Devices are organizations and professionals involved in emergency medical care and critical care pathways. Hospitals represent the largest segment of potential customers, specifically their emergency departments, intensive care units (ICUs), cardiac catheterization laboratories, and operating rooms. These departments frequently encounter patients experiencing cardiac arrest, and the consistent, high-quality CPR delivered by mechanical devices is invaluable during complex procedures, prolonged resuscitations, or when staff limitations necessitate automated assistance. Hospitals seek devices that are robust, easy to integrate with existing equipment, and provide reliable performance under high-stress conditions. The growing emphasis on improving patient outcomes and standardizing care protocols further drives hospital demand for these advanced tools.
Another significant segment of potential customers comprises Emergency Medical Services (EMS) providers, including ambulance services, fire departments with medical response capabilities, and air ambulance operations. For pre-hospital care, mechanical chest compression devices address critical challenges such as delivering effective CPR in confined spaces (e.g., ambulances), during patient movement, or in environments where manual CPR is difficult to sustain consistently. The ability to free up rescuers' hands for other critical interventions like defibrillation, airway management, or administering medications is a major advantage for EMS teams. Furthermore, military medical units and disaster relief organizations also represent vital potential customers, as they operate in challenging and dynamic environments where consistent, high-quality CPR is essential, and rescuer fatigue or safety can be significant concerns. Medical training and simulation centers also purchase these devices for educational purposes, ensuring future healthcare professionals are proficient in their use.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | 380 Million USD |
| Market Forecast in 2033 | 650 Million USD |
| Growth Rate | 8.0% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | ZOLL Medical Corporation (Stryker Corporation), Michigan Instruments, Schiller AG, Brunswick Medical, Physio-Control (Stryker), Cardiac Science (ZOLL), Defibtech (Stryker), Mindray Medical International Limited, SunLife Science, Becton, Dickinson and Company (BD), HeartSine Technologies (Stryker), Shanghai Boyuan Medical Instrument Co., Ltd., Vitatron, Resuscitation International, Intersurgical Ltd., Getinge AB, Shenzhen Comen Medical Instruments Co., Ltd., Nihon Kohden Corporation, C. R. Bard, Inc. (BD), Dragerwerk AG & Co. KGaA. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Mechanical Chest Compression Devices Market Key Technology Landscape
The technology landscape for the Mechanical Chest Compression Devices Market is characterized by continuous innovation aimed at enhancing device performance, user-friendliness, and integration with broader emergency medical systems. Core technologies revolve around the mechanical actuation mechanisms, primarily piston-driven systems and load-distributing band systems. Piston-driven devices typically utilize advanced electromechanical or pneumatic systems, incorporating precision motors, gear trains, or air compressors to deliver consistent and measured compressions. These systems often include sophisticated feedback loops with sensors that monitor compression depth, rate, and chest wall impedance, ensuring adherence to resuscitation guidelines. Load-distributing band devices, conversely, employ a wide band that wraps around the patient's chest, activated by a motor to apply circumferential pressure. Both types are continually refined for lighter weight, smaller footprint, and improved durability, making them more adaptable to diverse clinical scenarios.
Beyond the fundamental compression mechanisms, significant technological advancements are occurring in areas such as power management, data integration, and user interface design. Battery technology, particularly the adoption of high-capacity, fast-charging lithium-ion batteries, is crucial for extending operational time and enhancing portability for pre-hospital and transport applications. Devices are increasingly featuring advanced sensor arrays that provide real-time physiological data, such as end-tidal carbon dioxide (ETCO2) monitoring, which serves as a vital indicator of resuscitation quality and cardiac output. This sensor data is often processed by embedded microprocessors running sophisticated algorithms that optimize compression parameters. Wireless connectivity options, including Bluetooth and Wi-Fi, are becoming standard, enabling seamless data transfer to electronic health records (EHRs), patient monitors, and remote consultation platforms, thereby enhancing overall patient management and post-event analysis.
The evolution of user interfaces and the integration of artificial intelligence are also pivotal aspects of the current technology landscape. Modern mechanical chest compression devices feature intuitive graphical user interfaces (GUIs) with clear LCD or LED displays, guiding rescuers through setup and operation with minimal training required. Voice prompts and visual cues further enhance usability in high-stress environments. The nascent but rapidly growing application of AI and machine learning holds immense potential for future devices. AI algorithms can analyze complex physiological data to provide adaptive, patient-specific compression adjustments, predict resuscitation outcomes, and assist in real-time decision-making for clinicians. Furthermore, AI can contribute to predictive maintenance for the devices themselves, ensuring optimal functionality. These technological strides are not only improving the efficacy of resuscitation but also streamlining workflows and enhancing the overall safety and reliability of mechanical chest compression devices in critical emergency situations.
Regional Highlights
- North America: This region stands as a dominant force in the Mechanical Chest Compression Devices Market, driven by several key factors. The presence of a highly advanced healthcare infrastructure, coupled with robust emergency medical services networks, significantly contributes to high adoption rates. Strong awareness campaigns regarding the importance of high-quality CPR and the benefits of mechanical devices, often supported by organizations like the American Heart Association, have created a favorable market environment. Furthermore, consistent government funding for healthcare initiatives, coupled with favorable reimbursement policies, encourages hospitals and EMS providers to invest in these sophisticated devices. The region also benefits from a high concentration of key market players and a robust research and development ecosystem, fostering continuous innovation and product advancements tailored to clinical needs. The United States, in particular, leads in terms of market size and technological adoption, while Canada also shows strong and steady growth.
- Europe: The European market for mechanical chest compression devices is characterized by its maturity and stringent regulatory landscape. Countries across Western Europe, including Germany, France, the UK, and Scandinavia, exhibit high adoption due to well-established healthcare systems, an aging population more susceptible to cardiovascular diseases, and proactive public health initiatives. The European Resuscitation Council (ERC) guidelines play a significant role in promoting high-quality CPR, which often aligns with the capabilities of mechanical devices. While the market is mature, there remains consistent demand driven by replacement cycles, incremental technological advancements, and the expansion of EMS capabilities in Eastern European countries. Regulatory bodies like the European Medicines Agency (EMA) ensure high standards for device safety and efficacy, contributing to user confidence. Strategic collaborations between manufacturers and healthcare providers are common, facilitating the integration of these devices into standard emergency protocols.
- Asia Pacific (APAC): The Asia Pacific region is rapidly emerging as a high-growth market for mechanical chest compression devices, propelled by significant improvements in healthcare infrastructure, increasing disposable incomes, and a large and growing patient pool. Countries like China, India, Japan, and Australia are making substantial investments in modernizing their emergency medical services and critical care facilities. The rising prevalence of lifestyle-related diseases, including cardiovascular conditions, is leading to a higher incidence of sudden cardiac arrest, thereby fueling the demand for effective resuscitation tools. While awareness and adoption rates are still developing in some parts of the region compared to North America and Europe, government initiatives aimed at improving healthcare access and quality, coupled with a growing focus on medical tourism, are creating substantial opportunities for market expansion. Local manufacturing and distribution partnerships are key strategies for international players to penetrate this diverse and dynamic market.
- Latin America: The Latin American market for mechanical chest compression devices is in a nascent stage but shows promising growth potential. Economic development and increasing healthcare expenditure in countries such as Brazil, Mexico, and Argentina are gradually leading to the modernization of their emergency medical systems. Growing awareness among medical professionals about advanced resuscitation techniques and the benefits of mechanical devices is slowly driving adoption. However, challenges such as budget constraints, varying healthcare infrastructure quality, and the need for more comprehensive training programs continue to influence market penetration. International manufacturers are increasingly looking to establish a foothold in this region through local partnerships, competitive pricing strategies, and educational initiatives to overcome existing barriers and tap into the burgeoning demand for improved emergency medical care. The expansion of private healthcare facilities also contributes to market opportunities.
- Middle East and Africa (MEA): The Middle East and Africa region represents an evolving market for mechanical chest compression devices, characterized by significant disparities in healthcare development. Countries in the Gulf Cooperation Council (GCC) such as Saudi Arabia, UAE, and Qatar are investing heavily in state-of-the-art healthcare facilities and advanced medical technologies, driving demand for these devices. High healthcare spending, often government-backed, supports the adoption of premium medical equipment. In contrast, many parts of Africa face considerable challenges including limited healthcare budgets, underdeveloped infrastructure, and a shortage of skilled medical personnel. Despite these hurdles, there is growing interest and investment in emergency medical services development across the continent. Public health initiatives, supported by international organizations, are slowly improving awareness and access to basic and advanced life support tools, creating long-term growth prospects for mechanical chest compression devices in select sub-regions.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Mechanical Chest Compression Devices Market.- ZOLL Medical Corporation (Stryker Corporation)
- Michigan Instruments
- Schiller AG
- Brunswick Medical
- Physio-Control (Stryker)
- Cardiac Science (ZOLL)
- Defibtech (Stryker)
- Mindray Medical International Limited
- SunLife Science
- Becton, Dickinson and Company (BD)
- HeartSine Technologies (Stryker)
- Shanghai Boyuan Medical Instrument Co., Ltd.
- Vitatron
- Resuscitation International
- Intersurgical Ltd.
- Getinge AB
- Shenzhen Comen Medical Instruments Co., Ltd.
- Nihon Kohden Corporation
- C. R. Bard, Inc. (BD)
- Dragerwerk AG & Co. KGaA
Frequently Asked Questions
What are mechanical chest compression devices and how do they work?
Mechanical chest compression devices are automated medical instruments designed to deliver consistent, high-quality chest compressions during cardiopulmonary resuscitation (CPR) for patients experiencing sudden cardiac arrest. They work by using either a piston-based mechanism that applies direct pressure to the sternum or a load-distributing band that wraps around the chest to provide circumferential compressions. These devices are typically battery-powered and programmable to deliver compressions at specific depths and rates as recommended by resuscitation guidelines, minimizing rescuer fatigue and ensuring continuous, effective blood flow to vital organs.
What are the key advantages of using mechanical chest compression devices over manual CPR?
The primary advantages of mechanical chest compression devices include delivering highly consistent compression depth and rate, significantly reducing rescuer fatigue, particularly during prolonged resuscitation efforts or in challenging environments like moving ambulances. They minimize interruptions to compressions, which is critical for patient outcomes, and free up rescuers' hands to perform other life-saving interventions such as airway management, defibrillation, or medication administration. This consistency and efficiency can lead to improved patient survival rates and better neurological outcomes compared to manual CPR, especially when performed by fatigued or inexperienced personnel.
In which settings are these devices primarily used?
Mechanical chest compression devices are primarily used in various emergency and critical care settings. These include pre-hospital environments by Emergency Medical Services (EMS) personnel (e.g., in ambulances, at accident scenes) where patient transport or difficult conditions make manual CPR challenging. They are also widely utilized in hospitals, specifically in emergency departments, intensive care units (ICUs), cardiac catheterization laboratories, and during inter-facility patient transfers. Their ability to maintain continuous, high-quality CPR during complex medical procedures or extended resuscitation protocols makes them invaluable across diverse clinical scenarios.
What are the major challenges facing the mechanical chest compression devices market?
The mechanical chest compression devices market faces several challenges, with the high initial cost of these sophisticated devices being a significant barrier to widespread adoption, particularly in budget-constrained healthcare systems. Other challenges include the need for specialized training for operators to ensure correct and safe deployment, potential logistical complexities in rapid setup during critical emergencies, and some degree of resistance to change from traditional manual CPR methods. Furthermore, ensuring regulatory compliance across diverse international markets and managing concerns about potential device-related injuries, though rare with proper use, also pose hurdles for market growth.
How is artificial intelligence impacting the future of mechanical chest compression devices?
Artificial intelligence (AI) is poised to significantly impact the future of mechanical chest compression devices by enhancing their intelligence and adaptability. AI algorithms can process real-time patient physiological data (e.g., ECG, ETCO2) to dynamically adjust compression parameters (depth, rate, recoil), providing truly personalized and optimal CPR. This adaptive capability moves beyond fixed protocols, aiming for maximum blood flow and improved outcomes. Additionally, AI can improve training through advanced simulations, offer predictive analytics for patient outcomes, facilitate seamless integration with broader life support systems, and optimize device maintenance, ultimately making resuscitation efforts more precise, efficient, and effective.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager