Medical Cautery Pen Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 436452 | Date : Dec, 2025 | Pages : 242 | Region : Global | Publisher : MRU
Medical Cautery Pen Market Size
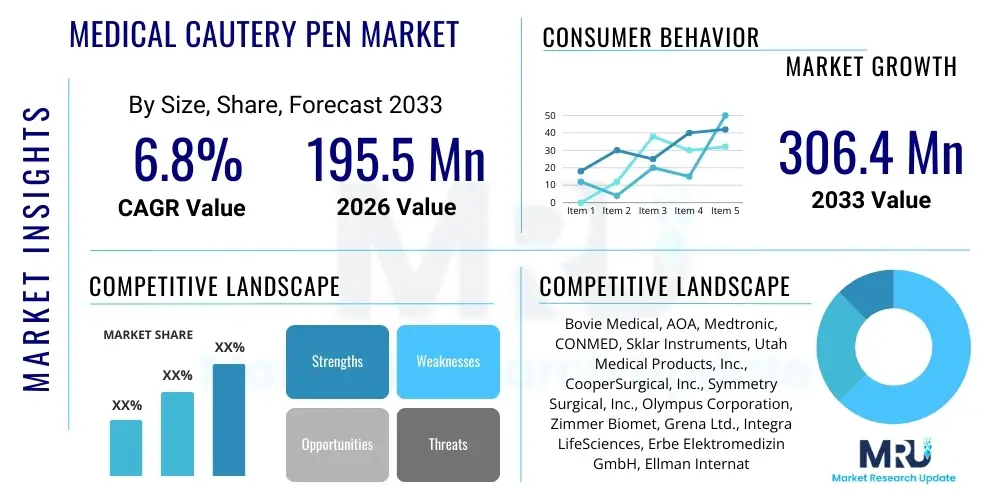
The Medical Cautery Pen Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2026 and 2033. The market is estimated at USD 195.5 Million in 2026 and is projected to reach USD 306.4 Million by the end of the forecast period in 2033.
Medical Cautery Pen Market introduction
The Medical Cautery Pen Market encompasses sophisticated handheld electrosurgical devices utilized primarily for achieving hemostasis and precision tissue ablation through the application of heat. These devices are predominantly single-use, battery-powered instruments designed for maximum portability and ease of sterilization management, addressing critical surgical needs across various clinical settings. The market's growth trajectory is strongly influenced by the increasing volume of minimally invasive surgical procedures, especially in outpatient and ambulatory surgical centers (ASCs), where quick setup and disposable safety are paramount. Cautery pens, often referred to as electrocautery or thermal pens, offer controlled localized heating, significantly reducing blood loss and improving surgical field visibility, thus contributing to faster procedure times and enhanced patient outcomes.
The primary function of these specialized pens is coagulation, cutting, or fulguration of tissue, making them indispensable in specialties such as dermatology, plastic surgery, ophthalmology, and minor general surgery. Key product categories include high-temperature cauterizers used for heavier coagulation tasks and low-temperature models preferred for fine, delicate procedures, particularly in neurosurgery or ophthalmic applications where thermal spread minimization is critical. Continuous innovation focuses on improving battery life, ergonomic design, and integrating precise temperature control mechanisms to enhance surgical accuracy and reduce collateral tissue damage. The transition from traditional reusable devices, which require cumbersome sterilization cycles, towards sterile, ready-to-use disposable pens is a major market driver, aligning with heightened infection control standards globally.
Major applications driving market demand include the removal of warts, skin tags, and minor lesions in dermatological clinics, precise vessel sealing in reconstructive surgery, and management of bleeding during biopsies. The robust adoption of these pens is further supported by the growing elderly population worldwide, which necessitates a higher volume of surgical interventions. Furthermore, the economic benefits derived from disposable instruments—eliminating reprocessing costs and associated regulatory burdens—make them an attractive option for healthcare providers aiming for operational efficiency. Market participants are strategically focusing on geographical expansion and product diversification, introducing specialized tips and variable power settings to cater to niche surgical requirements, solidifying the cautery pen's role as a staple tool in modern surgical practice.
Medical Cautery Pen Market Executive Summary
The Medical Cautery Pen Market is experiencing robust growth driven by the shifting preference towards disposable, sterile electrosurgical tools and the expanding infrastructure of Ambulatory Surgical Centers (ASCs). Business trends indicate a strong focus on high-temperature pens due to their broad utility in general surgical procedures, while low-temperature pens are gaining traction in highly specialized fields requiring meticulous thermal management. Investment in R&D is concentrated on developing pens with enhanced battery stability and ergonomic features, alongside compliance with stringent regulatory frameworks concerning single-use medical devices. Key market players are engaging in strategic partnerships and acquisitions to secure distribution channels and technological expertise, ensuring competitive pricing and broad market penetration across developing and mature economies.
Regionally, North America maintains the dominant market share, characterized by high healthcare expenditure, sophisticated surgical infrastructure, and rapid adoption of advanced disposable technologies. However, the Asia Pacific (APAC) region is projected to exhibit the fastest growth rate, fueled by improving healthcare access, increasing medical tourism, and rising awareness regarding the benefits of minimally invasive procedures. Governments in APAC are also investing heavily in upgrading hospital infrastructure, which directly translates into increased demand for essential surgical instruments like cautery pens. Europe remains a significant market, driven by favorable reimbursement policies and a strong emphasis on reducing hospital-acquired infections (HAIs), making disposable solutions highly attractive.
Segmentation trends highlight the supremacy of the Hospital segment in terms of revenue, although ASCs are emerging as the fastest-growing end-user segment due to the cost-effectiveness and efficiency they offer for minor and intermediate procedures. Product segment analysis confirms the high-temperature pens segment's current lead due to versatility in general surgery, but the specialized demand for low-temperature pens, particularly in cosmetic and neuro-micro-cautery, ensures their continued high-value growth. The underlying market dynamic emphasizes convenience, sterilization assurance, and precision, dictating product development strategies focused on offering reliable, portable, and disposable cauterization solutions that minimize surgical risks and improve workflow in dynamic clinical environments.
AI Impact Analysis on Medical Cautery Pen Market
User inquiries regarding the integration of Artificial Intelligence (AI) in the Medical Cautery Pen Market primarily revolve around how AI can enhance the precision, safety, and training associated with electrosurgery, given that the cautery pen itself is a non-smart, single-use physical tool. Key themes analyzed include the use of AI in predicting tissue response to thermal energy, optimizing power settings based on real-time feedback, and developing sophisticated surgical simulation environments. Users express interest in whether AI can contribute to 'smarter' disposable instruments or if its impact is limited to surrounding technologies like advanced monitoring systems, surgical robots, and procedural documentation. Concerns often center on the practical applicability and cost-effectiveness of integrating complex AI algorithms with low-cost disposable devices, alongside expectations for AI-driven post-operative analysis to monitor wound healing related to cauterization.
AI's influence, while not directly embedded in the disposable pen itself (due to cost and disposable nature), significantly impacts the context and precision of electrosurgery. Machine learning algorithms are being trained on vast datasets of tissue impedance and thermal diffusion characteristics to create predictive models that guide surgeons on optimal power and duration settings, which are critical for minimizing thermal injury when using a cautery pen. This 'AI-enhanced guidance' system, often integrated into adjacent electrosurgical generators or navigation platforms, provides a layer of intelligence that compensates for variations in tissue type and moisture content, thus improving the functional performance of even simple disposable pens. Furthermore, AI is crucial in simulating complex surgical scenarios, allowing trainees to practice precise movements and cauterization techniques without risk, thereby standardizing the skill level across the surgical workforce and reducing reliance on manual dexterity alone during live procedures.
The future trajectory suggests AI-powered quality control during the manufacturing process of the pens, ensuring consistency in tip material and heating elements. More significantly, AI is expected to revolutionize post-procedure documentation by automatically analyzing video feeds of the surgical site, documenting the exact points and duration of cauterization, which is invaluable for regulatory compliance, quality assurance, and legal records. Though the cautery pen remains simple, AI enhances the entire procedure lifecycle—from pre-operative planning and intra-operative guidance to post-operative reporting—driving demand for devices that are compatible with advanced visualization and feedback systems, pushing manufacturers to ensure material purity and consistent thermal output across all disposable units.
- AI optimizes real-time power delivery recommendations in electrosurgical units paired with cautery pens, enhancing precision.
- Predictive modeling using AI assists in minimizing collateral thermal damage by analyzing tissue characteristics.
- Advanced AI-driven surgical simulators provide realistic training for precise cautery pen application, improving surgical competency.
- AI aids in automated quality assurance during the high-volume manufacturing of disposable cautery pen components.
- Machine learning algorithms are utilized for post-operative analysis of surgical outcomes related to hemostasis achieved by cautery.
- AI integration into surgical navigation systems helps guide the trajectory and depth of cauterization in complex procedures.
DRO & Impact Forces Of Medical Cautery Pen Market
The Medical Cautery Pen Market is fundamentally shaped by several powerful drivers (D) such as the increasing global prevalence of minimally invasive surgeries (MIS) and the high demand for disposable tools to ensure stringent infection control. The primary restraints (R) include intense price competition, the regulatory complexities associated with single-use medical devices, and the risk of substitution by advanced electrosurgical generators with reusable handpieces. Significant opportunities (O) lie in expanding market penetration into emerging economies with developing healthcare infrastructure, and the continuous innovation in material science to produce more energy-efficient and specialized thermal tips. These elements interact through core impact forces, predominantly driven by technological advancement in power sources (e.g., high-density miniature batteries) and the overwhelming global mandate for patient safety through disposability, which sustains the market's upward trajectory despite pricing pressures.
Drivers (D): The surging preference for outpatient settings, such as Ambulatory Surgical Centers (ASCs), heavily favors disposable cautery pens due to their operational convenience, eliminating the need for complex in-house sterilization processes and associated capital investment. The aging global demographic contributes substantially to the volume of surgical procedures requiring precise hemostasis, including dermatological, ocular, and minor vascular surgeries, where the portability and immediate readiness of cautery pens are critical advantages. Furthermore, governmental and organizational efforts worldwide to curb the incidence of hospital-acquired infections (HAIs) rigorously promote the use of single-use instruments, directly accelerating the adoption of disposable cautery pens across all healthcare segments. The continuous technological refinement leading to more specialized tips for intricate procedures also expands the clinical utility of these devices.
Restraints (R): A significant restraint is the high volume of medical waste generated by single-use disposable instruments, leading to environmental concerns and rising waste disposal costs for healthcare facilities, especially in regions with strict waste management regulations. Furthermore, the intense competition among manufacturers, particularly from Asian suppliers, results in aggressive pricing strategies that compress profit margins, challenging smaller market players and limiting substantial investment in premium features. Another critical challenge is the potential for thermal damage to surrounding healthy tissue if the pen is improperly used or if its temperature control mechanism fails, necessitating strict training protocols and reliable quality control, which adds overhead complexity to device deployment.
Opportunities (O): Emerging markets, particularly in Asia Pacific and Latin America, present vast untapped potential as disposable medical device usage increases alongside healthcare infrastructure development and rising middle-class income. Technological advancements focusing on integrating smart features, such as audible feedback mechanisms or enhanced ergonomic designs that reduce surgeon fatigue, offer lucrative avenues for differentiation and premium pricing. Moreover, expanding the application scope into minimally invasive aesthetic and reconstructive procedures, which demand high precision and predictable outcomes, represents a stable high-growth opportunity for specialized low-temperature cautery pens.
Impact Forces: The overarching regulatory scrutiny on disposable medical devices, demanding robust verification of sterility and consistent performance, acts as a pivotal force. Economic forces, driven by the shift from expensive reusable equipment maintenance to lower per-procedure costs associated with disposables, continue to favor market expansion. Technological forces, particularly in battery miniaturization and tip material engineering (e.g., non-stick coatings), consistently improve the user experience and clinical efficacy, making the devices safer and more reliable. Finally, societal pressures for faster recovery times and reduced surgical invasiveness inherently support the tools designed for efficiency and precise control, such as high-quality cautery pens.
Segmentation Analysis
The Medical Cautery Pen Market is comprehensively segmented based on Product Type, Application, and End-User, providing a detailed view of demand dynamics across the healthcare ecosystem. The Product Type segmentation distinguishes between High-Temperature Cautery Pens and Low-Temperature Cautery Pens, reflecting the distinct clinical needs for coagulation power and precision. High-temperature pens typically dominate the volume segment due to their general applicability in achieving rapid hemostasis during procedures ranging from skin biopsies to general surgery. Conversely, low-temperature pens, designed for meticulous control and minimal thermal spread, command a premium and are critical in specialized applications such as ophthalmology and neurosurgery.
Analysis by Application reveals the widespread utility of these devices, with Dermatology and General Surgery segments consistently generating the largest revenue due to the high volume of routine procedures such as mole and wart removals, and minor incision control. The burgeoning cosmetic surgery segment also significantly contributes to revenue, demanding aesthetically precise coagulation tools. The End-User segmentation provides crucial insights into consumption patterns, with Hospitals holding the largest market share owing to the high volume of complex surgeries performed. However, the rapidly expanding Ambulatory Surgical Centers (ASCs) segment is recognized as the fastest-growing area, driven by efficiency, lower operating costs, and the increasing migration of less complex procedures from inpatient settings to outpatient facilities, favoring disposable and portable cautery solutions.
Understanding these segments is essential for manufacturers and distributors to tailor their product offerings and market strategies effectively. For instance, focusing marketing efforts for low-temperature, fine-tipped pens toward specialized ophthalmic clinics and neurosurgical units, while positioning standard, cost-effective high-temperature pens for bulk procurement by large hospital networks or ASC chains, ensures optimal market penetration. The continuous evolution of surgical techniques towards minimally invasive approaches across all application segments will further solidify the need for specialized, easy-to-use, disposable hemostatic tools, reinforcing the importance of granular segmentation analysis for strategic planning.
- Product Type:
- High-Temperature Cautery Pens
- Low-Temperature Cautery Pens
- Application:
- Dermatology
- Ophthalmology
- Gynecology
- Cardiac Surgery
- Plastic and Reconstructive Surgery
- General Surgery
- Neurosurgery
- End-User:
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics
- Physician Offices
Value Chain Analysis For Medical Cautery Pen Market
The value chain for the Medical Cautery Pen Market is characterized by a high reliance on specialized material sourcing and rigorous quality control during manufacturing, particularly given the product’s disposable and safety-critical nature. The upstream activities involve the procurement of highly specific materials, including medical-grade plastics for the housing, high-performance alloys for the cautery tips, and crucial miniature, high-density batteries. Suppliers of these core components must adhere to strict quality standards and regulatory certifications (such as ISO 13485). The efficiency and cost of battery procurement significantly influence the final product price, compelling manufacturers to maintain strong, long-term relationships with specialized component providers to ensure consistent supply and competitive pricing, forming the foundation of the value chain's stability.
Midstream, the manufacturing process is highly specialized, involving precision molding, electronic assembly (connecting the battery, switch, and heating element), and critical quality assurance checks on temperature consistency and tip performance. Sterilization is a mandatory and capital-intensive step, typically achieved through ethylene oxide (EtO) or radiation sterilization, impacting production throughput and cost. Downstream activities involve distribution channels, which are crucial for reaching diverse end-users. Direct channels are often utilized for large hospital systems or governmental contracts, providing greater control over pricing and customer feedback. Indirect distribution, leveraging national and regional medical device distributors, is vital for accessing smaller specialty clinics, ASCs, and individual physician offices, requiring effective margin sharing and strong inventory management systems to ensure product availability.
The final stage involves the service and end-use interaction. While the product is disposable, the successful adoption heavily relies on effective training and technical support provided by manufacturers or distributors, ensuring clinicians understand the optimal use of various tips and temperature settings. The feedback loop from surgeons to manufacturers is invaluable for continuous product improvement, especially concerning ergonomic design and functional precision. The efficiency of the entire value chain is measured by its ability to deliver a consistently sterile, reliable, and cost-effective product rapidly to the point of care, thus minimizing logistical bottlenecks and maximizing clinical uptake across global markets.
Medical Cautery Pen Market Potential Customers
The primary end-users and potential customers for medical cautery pens are diverse surgical and clinical facilities characterized by a high volume of minor and intermediate procedures requiring controlled hemostasis. Hospitals, specifically those operating emergency departments, outpatient clinics, and specialized surgical suites (such as ophthalmic or gynecological), represent the largest current customer base. These institutions value the immediate availability, guaranteed sterility, and standardized performance that disposable pens offer, crucial for reducing surgical turnaround times and managing multi-department inventory efficiently. Large hospital networks often seek bulk purchasing agreements, focusing on pens that offer a robust combination of performance and cost-effectiveness across general surgical requirements.
Ambulatory Surgical Centers (ASCs) and Specialty Clinics are rapidly growing customer segments, highly valuing portability and the elimination of sterilization overhead. ASCs, which focus on procedures that do not require overnight stays (e.g., endoscopy, cataract surgery, cosmetic procedures), are ideally suited for disposable cautery pens. Their operational model prioritizes efficiency and cost control, making single-use instruments an attractive proposition compared to maintaining and reprocessing complex electrosurgical units. These centers typically require fine-tipped, low-temperature pens for cosmetic and minimally invasive procedures where aesthetic outcome is paramount, demanding precise control over thermal spread.
Furthermore, individual physician offices, particularly those specializing in dermatology, podiatry, and veterinary medicine, form another substantial segment. For these smaller practices, the cautery pen serves as a highly convenient, self-contained, and space-saving alternative to full electrosurgical generators, allowing them to perform minor surgical interventions directly in the office setting. Dermatologists use them extensively for the removal of benign lesions, demanding ease of use and consistent results. These diverse customer needs necessitate a comprehensive product portfolio from manufacturers, ranging from basic, cost-optimized pens for high-volume settings to highly specialized pens for precision-driven specialty clinics, all while adhering to strict safety and sterilization protocols.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 195.5 Million |
| Market Forecast in 2033 | USD 306.4 Million |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Bovie Medical, AOA, Medtronic, CONMED, Sklar Instruments, Utah Medical Products, Inc., CooperSurgical, Inc., Symmetry Surgical, Inc., Olympus Corporation, Zimmer Biomet, Grena Ltd., Integra LifeSciences, Erbe Elektromedizin GmbH, Ellman International, Stryker Corporation, B. Braun Melsungen AG, Teleflex Incorporated, Kirwan Surgical Products, Geister Medizintechnik GmbH, Virox Technologies Inc. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Medical Cautery Pen Market Key Technology Landscape
The technological landscape of the Medical Cautery Pen Market, though seemingly simple, is characterized by continuous refinement focusing on power efficiency, thermal consistency, and specialized tip design. The primary technology revolves around resistive heating elements, typically high-resistance wire loops or filaments, housed within a precision tip. Innovation is heavily concentrated on the power source—the miniaturized battery technology. Manufacturers are adopting higher energy density lithium batteries or specialized alkaline variants to ensure maximum duration of operational heat while maintaining a compact, lightweight form factor. This focus is critical as insufficient battery life is a major complaint in single-use devices, directly impacting surgical workflow and reliability. Furthermore, advances in polymer science are enabling the construction of ergonomic, heat-resistant housing materials that improve grip and thermal insulation, ensuring user comfort and safety during prolonged use.
A key area of technological differentiation lies in the development of specialized cautery tips. Modern cautery pens feature tips made from materials like nickel-chromium or stainless steel, often with specialized non-stick coatings (e.g., PTFE or similar polymers) to prevent tissue adherence, which is crucial for clean excision and coagulation, particularly in ophthalmic or cosmetic surgery. The geometry of these tips is continuously being optimized—ranging from fine pointed tips for meticulous procedures to large loop tips for broader coagulation areas. Low-temperature pens, in particular, rely on highly sophisticated internal temperature control circuitry, sometimes incorporating micro-thermistors, to maintain a precise temperature range, minimizing the risk of unwanted charring or excessive thermal spread to adjacent healthy tissue, enhancing overall surgical precision and predictability.
Safety features constitute another major technological focus. This includes robust internal mechanisms to prevent accidental activation (safety switches) and highly stable thermal regulation systems that prevent overheating, ensuring the device remains within certified temperature limits throughout its operational life. The integration of high-reliability switching mechanisms that can handle the high current demands of the resistive element without failing is also paramount. Ultimately, the technology landscape is driven by the mandate to deliver consistent, predictable energy output in a sterile, disposable package, demanding rigorous engineering in miniaturization, power management, and material selection to meet the evolving clinical standards for electrosurgery precision and infection control.
Regional Highlights
- North America: North America, led by the United States, commands the largest share of the Medical Cautery Pen Market, primarily due to established healthcare infrastructure, high per capita healthcare spending, and widespread adoption of disposable medical technology. The region benefits from favorable reimbursement policies and a strong regulatory environment (FDA approval) that promotes the use of single-use devices to reduce HAIs. The increasing number of outpatient surgical procedures performed in ASCs further accelerates demand. Market saturation in terms of basic product usage drives companies here to focus on premium, specialized products, such as precise low-temperature pens for cosmetic and ophthalmic procedures, ensuring continued revenue growth despite market maturity.
- Europe: The European market represents a significant revenue contributor, supported by high standards of surgical care and government initiatives aimed at maximizing patient safety and minimizing cross-contamination risks. Countries like Germany, France, and the UK are major consumers, emphasizing quality and environmental compliance. While the market is mature, growth is sustained by the ongoing transition from reusable to disposable equipment and the increasing adoption of minimally invasive techniques across surgical specialties.
- Asia Pacific (APAC): APAC is projected to be the fastest-growing regional market, driven by rapid improvements in healthcare access, significant investments in hospital infrastructure, and growing medical tourism in countries such as China, India, and South Korea. Rising disposable incomes and increasing awareness of advanced surgical methods are boosting the demand for affordable, reliable disposable instruments. The large patient pool and less restrictive regulatory pathways (compared to the US/EU) for market entry make this region highly attractive for global manufacturers seeking volume expansion.
- Latin America (LATAM): The LATAM region shows steady growth, primarily driven by expanding healthcare coverage, particularly in Brazil and Mexico. Economic volatility and pricing sensitivity remain key factors, leading to a strong demand for cost-effective, high-volume cautery pens. Market penetration is gradually increasing as local governments and private clinics prioritize standardized disposable solutions to elevate safety standards in basic surgical care.
- Middle East and Africa (MEA): The MEA market growth is concentrated in the Gulf Cooperation Council (GCC) countries (UAE, Saudi Arabia) due to high healthcare spending, rapid modernization of medical facilities, and reliance on international surgical standards. In Africa, market adoption is slower, often limited to major metropolitan areas, but the push for better infection control provides a foundational driver for single-use devices.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Medical Cautery Pen Market.- Bovie Medical
- AOA
- Medtronic
- CONMED
- Sklar Instruments
- Utah Medical Products, Inc.
- CooperSurgical, Inc.
- Symmetry Surgical, Inc.
- Olympus Corporation
- Zimmer Biomet
- Grena Ltd.
- Integra LifeSciences
- Erbe Elektromedizin GmbH
- Ellman International
- Stryker Corporation
- B. Braun Melsungen AG
- Teleflex Incorporated
- Kirwan Surgical Products
- Geister Medizintechnik GmbH
- Virox Technologies Inc.
Frequently Asked Questions
Analyze common user questions about the Medical Cautery Pen market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary difference between high-temperature and low-temperature cautery pens?
High-temperature cautery pens typically operate above 1,000°C and are utilized for rapid coagulation and cutting in general surgery. Low-temperature pens operate between 120°C and 450°C, offering highly precise thermal control crucial for delicate procedures in ophthalmology or neurosurgery where minimizing thermal spread is paramount.
Why are disposable cautery pens favored over reusable electrosurgical units in ASCs?
Ambulatory Surgical Centers (ASCs) favor disposable pens because they eliminate the need for costly sterilization protocols, reduce the risk of cross-contamination (HAIs), and improve workflow efficiency by providing a sterile, ready-to-use device immediately, resulting in lower overall operational expenses per procedure.
Which geographical region exhibits the highest growth potential for the Medical Cautery Pen Market?
The Asia Pacific (APAC) region is projected to register the fastest growth rate. This acceleration is driven by significant infrastructure investments in healthcare, increasing patient access to surgical care, and a rapid shift towards disposable medical supplies to meet emerging international safety standards.
What are the main regulatory challenges faced by manufacturers in this market?
Manufacturers face stringent regulatory hurdles, particularly in North America and Europe, centered around proving the sterility, consistent thermal output, and biocompatibility of the single-use components, alongside managing the increasing environmental concerns related to medical waste disposal generated by disposable products.
How is technology currently influencing the performance and design of cautery pens?
Technology is driving innovation in power management (miniaturized, high-density batteries), material science (non-stick tips and ergonomic housing), and specialized tip geometry, focusing on improving precision, ensuring stable temperature delivery, and enhancing the overall safety and ease of use for the operating clinician.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
