Medical DVT Pumps Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 431477 | Date : Dec, 2025 | Pages : 248 | Region : Global | Publisher : MRU
Medical DVT Pumps Market Size
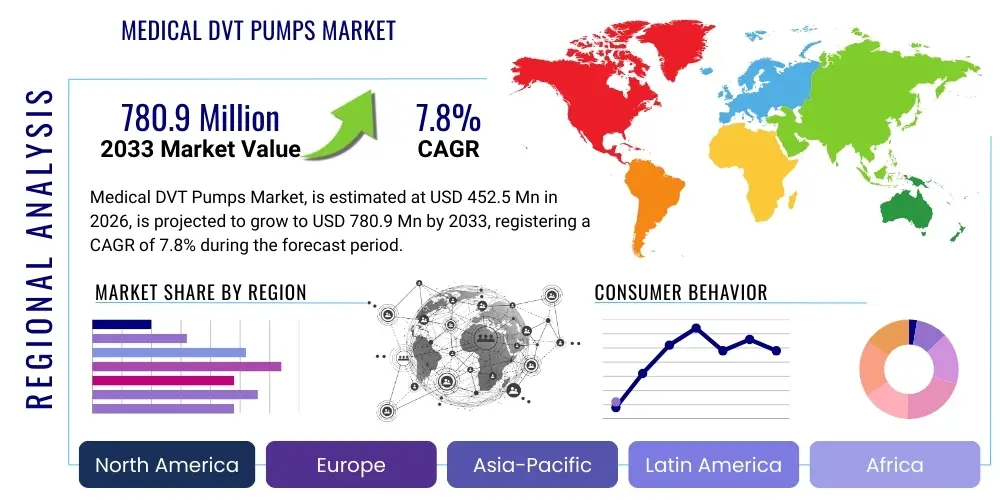
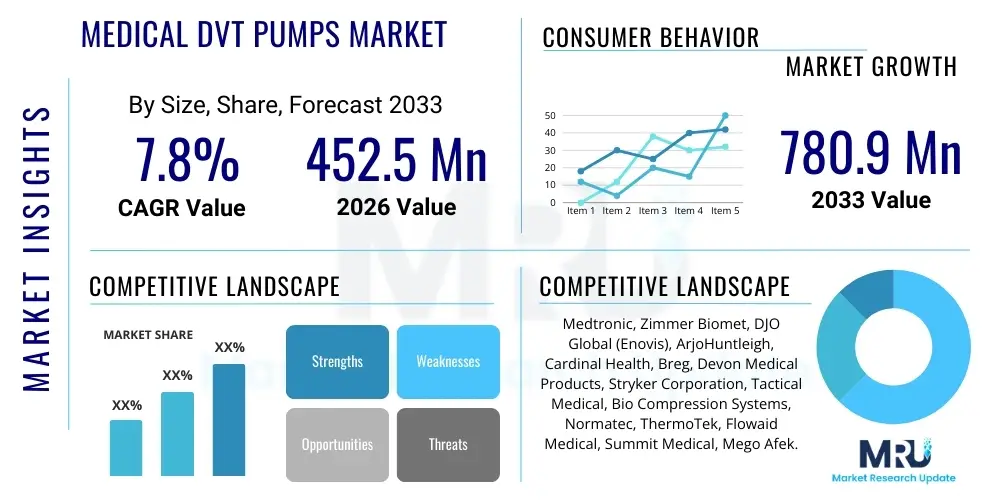
The Medical DVT Pumps Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.8% between 2026 and 2033. The market is estimated at USD 452.5 Million in 2026 and is projected to reach USD 780.9 Million by the end of the forecast period in 2033.
Medical DVT Pumps Market introduction
The Medical Deep Vein Thrombosis (DVT) Pumps Market encompasses devices engineered to provide prophylaxis against DVT, a condition characterized by blood clot formation, typically in the deep veins of the legs. These devices, primarily utilizing Intermittent Pneumatic Compression (IPC) technology, function by sequentially inflating and deflating air chambers wrapped around the patient's limbs, mimicking natural muscle contraction. This action enhances venous blood flow and prevents venous stasis, significantly lowering the risk of life-threatening pulmonary embolism (PE). The core product involves a pneumatic controller unit, tubing, and single-use or reusable compression garments designed for various anatomical sites, including the calf, thigh, and foot.
Major applications for DVT pumps include high-risk surgical settings, such as orthopedic, general, and bariatric procedures, where patient immobility is prolonged. They are also crucial in critical care units, post-operative recovery wards, and long-term care facilities for patients with reduced mobility due to chronic illness or trauma. The primary benefits of using DVT pumps include non-invasive clot prevention, reduced reliance on pharmacological anticoagulants (which carry bleeding risks), and suitability for patients contraindicated for chemical prophylaxis. These factors underscore their essential role in contemporary patient safety protocols and hospital accreditation standards.
Driving factors propelling the market growth include the escalating prevalence of chronic diseases like obesity, diabetes, and cardiovascular disorders, which heighten DVT risk. Furthermore, the global aging population, coupled with an increasing volume of complex surgical procedures requiring extended post-operative immobilization, generates consistent demand. Enhanced clinical awareness and the establishment of robust institutional protocols mandating mechanical prophylaxis are also significant catalysts. Technological advancements leading to more portable, user-friendly, and clinically efficacious devices, particularly those integrating smart monitoring and compliance tracking capabilities, further solidify market expansion.
Medical DVT Pumps Market Executive Summary
The Medical DVT Pumps Market demonstrates robust growth driven by mandatory surgical prophylaxis guidelines and continuous technological innovation focusing on enhancing patient compliance and portability. Current business trends indicate a shift towards advanced, non-tethered devices and integrated smart technology for real-time patient monitoring, which allows healthcare providers to track usage patterns and ensure therapeutic efficacy, particularly in high-acuity settings and increasingly, in home care. Key manufacturers are prioritizing the development of highly efficient, noiseless pumps and single-patient use garments to minimize infection risk and streamline clinical workflows. Strategic mergers, acquisitions, and partnerships aimed at expanding distribution networks and integrating DVT prevention into broader post-acute care solutions define the competitive landscape.
Regional trends highlight North America's dominance, attributed to well-established healthcare infrastructure, high procedural volumes, favorable reimbursement policies for DVT prophylaxis, and stringent guidelines from regulatory bodies like the Centers for Disease Control and Prevention (CDC). However, the Asia Pacific (APAC) region is poised for the fastest expansion, fueled by improving healthcare access, rising disposable incomes, rapid urbanization leading to increased chronic disease burden, and significant investments in modernizing hospital infrastructure, particularly in emerging economies like China and India. Europe maintains a steady growth trajectory, supported by universal healthcare coverage and high adoption rates of evidence-based medicine.
Segmentation trends confirm the intermittent pneumatic compression (IPC) device segment's stronghold due to its clinical proven efficacy and widespread acceptance. Within end-users, hospitals remain the largest revenue generator owing to the high concentration of surgical procedures and critical care admissions; however, the fastest growth is anticipated within the home care settings segment. This surge is directly linked to the paradigm shift towards early patient discharge and the necessity for continued DVT management in the comfort of a patient's residence. The development of specialized, affordable, and easy-to-operate devices tailored for home use is accelerating this transition, significantly influencing market dynamics and product pipeline development.
AI Impact Analysis on Medical DVT Pumps Market
User queries regarding the intersection of Artificial Intelligence (AI) and the Medical DVT Pumps Market predominantly revolve around improving risk stratification, enhancing patient adherence, and optimizing device performance. Users frequently ask if AI can predict which patients require prolonged mechanical prophylaxis beyond standard guidelines, how AI-driven monitoring systems can detect suboptimal fit or usage errors in real-time, and whether predictive analytics can be integrated into electronic health records (EHRs) to automate DVT risk assessments. The key themes summarized from these concerns highlight the expectation that AI should transition DVT prevention from a standardized procedural requirement to a personalized, adaptive therapeutic intervention. Concerns also include data privacy, the cost of implementing AI-enabled devices, and the accuracy of algorithms interpreting complex patient physiological data points, particularly in diverse clinical settings.
AI's influence is expected to dramatically enhance the operational effectiveness and clinical outcomes associated with DVT prevention devices. By integrating machine learning algorithms with data streams from patient monitors and the DVT pump usage logs, AI can create highly accurate, individualized risk profiles. This permits clinicians to tailor compression cycles, duration, and intensity specific to the patient’s hemodynamic response and underlying comorbidities, moving beyond fixed treatment protocols. Furthermore, AI-powered systems can analyze vast datasets of surgical and medical histories to proactively flag patients transitioning between care settings, ensuring continuity of prophylaxis and reducing instances of preventable DVT.
The implementation of sophisticated sensor technology combined with AI allows for continuous, non-invasive monitoring of limb circumference and skin perfusion, adjusting the compression pattern dynamically. This significantly addresses the critical issue of patient compliance, as algorithms can identify and alert both the patient and the care team if the device is incorrectly worn, disconnected, or malfunctioning. This level of smart intervention not only maximizes the therapeutic benefit of the pump but also provides quantifiable data on usage adherence, which is vital for quality reporting and value-based care initiatives, ultimately reducing overall hospital readmission rates related to venous thromboembolism (VTE).
- AI-driven personalized risk assessment based on EHR data and real-time physiological metrics.
- Enhanced compliance monitoring through machine learning algorithms detecting and correcting improper device usage or fit.
- Predictive maintenance alerts for DVT pump hardware, optimizing clinical availability and reliability.
- Integration of smart algorithms to dynamically adjust pneumatic compression cycles based on patient movement and hemodynamic changes.
- Optimization of inventory and logistics management in hospitals through AI-forecasting of device demand based on surgical schedules.
DRO & Impact Forces Of Medical DVT Pumps Market
The Medical DVT Pumps market is shaped by a confluence of influential factors, categorized as drivers, restraints, and opportunities (DRO), which collectively determine market trajectory and competitive intensity. The primary drivers are the globally increasing incidence of VTE risk factors, including the expanding elderly population and the rising number of complex orthopedic and abdominal surgeries requiring extended periods of immobility. Mandates and strong clinical evidence supporting mechanical prophylaxis, particularly in settings where pharmacological agents are contraindicated, provide an indispensable demand floor for these devices. Furthermore, continuous product evolution leading to advanced, ergonomic, and patient-friendly designs, such as tubeless and portable units, significantly enhances patient compliance and facilitates market penetration into non-traditional care settings.
Conversely, market expansion is constrained by several crucial factors. The primary restraint involves the comparatively high cost associated with advanced IPC devices, particularly the single-patient use garments, which contribute substantially to recurring operational expenses for healthcare facilities. Additionally, challenges related to ensuring high patient compliance, especially in outpatient and home-care settings, persist despite technological improvements. Furthermore, the lack of standardized, global reimbursement policies and the dominance of pharmacological prophylaxis (anticoagulants) in certain clinical scenarios pose competitive hurdles. Healthcare professionals sometimes overlook mechanical prophylaxis or prioritize cheaper, less effective methods, particularly in resource-constrained environments.
Opportunities for growth are abundant, primarily centered on expanding product application beyond traditional post-surgical recovery to areas such as chronic wound management and high-risk mobility patients in nursing homes. The most compelling opportunity lies in the development of highly integrated, IoT-enabled DVT pumps that communicate usage data directly to electronic health records (EHRs), streamlining administrative burden and improving continuity of care. Furthermore, market penetration into rapidly developing economies in APAC and Latin America, facilitated by localized manufacturing and tailored pricing strategies, offers substantial untapped potential. The COVID-19 pandemic also highlighted the critical need for VTE prevention in acutely ill patients, opening new avenues for pump usage in critical care medicine.
Segmentation Analysis
The Medical DVT Pumps Market is meticulously segmented based on Product Type, End-User, and Application, providing a granular view of market dynamics and demand distribution. This segmentation is crucial for stakeholders to identify high-growth areas and tailor product development and marketing strategies effectively. The product landscape is dominated by Intermittent Pneumatic Compression (IPC) devices, which are highly preferred across institutional settings due to their clinically proven efficacy in mimicking physiological blood flow. However, smaller, specialized segments like venous foot pumps also contribute, offering localized compression for specific patient profiles. The sophistication and disposability of the accompanying garments further segment the product type, impacting recurring revenue streams for manufacturers.
The End-User analysis reveals that hospitals, encompassing large tertiary care centers and smaller community hospitals, represent the largest consumer base, driven by the high volume of surgical procedures performed and the strict adherence to DVT prophylaxis protocols. Nonetheless, the market is experiencing a rapid structural shift towards non-institutional settings. Ambulatory Surgical Centers (ASCs) are increasingly utilizing DVT pumps due to the rising complexity of same-day surgical procedures, while the Home Care segment is emerging as the fastest-growing category. This accelerated growth in non-institutional settings is facilitated by the introduction of compact, easily manageable, and cost-effective devices suitable for patient self-administration.
Application segmentation primarily categorizes usage into DVT Prophylaxis and Chronic Venous Insufficiency (CVI) or Lymphedema treatment. Prophylaxis remains the core application, spanning pre-operative, intra-operative, and post-operative care across various medical specialties. However, the use of DVT pump technology, often adapted into more powerful sequential compression devices, is expanding into chronic disease management, particularly for reducing limb swelling and improving circulation in patients suffering from severe CVI or secondary lymphedema. This expansion broadens the addressable market beyond acute surgical settings, tapping into long-term patient care needs and diversifying revenue sources for market participants.
- By Product Type:
- Intermittent Pneumatic Compression (IPC) Devices
- Venous Foot Pumps
- Compression Garments (Disposable vs. Reusable)
- By End-User:
- Hospitals and Clinics
- Ambulatory Surgical Centers (ASCs)
- Home Care Settings
- Long-Term Care Facilities
- By Application:
- Deep Vein Thrombosis (DVT) Prophylaxis
- Lymphedema Treatment
- Chronic Venous Insufficiency Management
Value Chain Analysis For Medical DVT Pumps Market
The value chain for the Medical DVT Pumps market is characterized by several distinct stages, beginning with sophisticated upstream manufacturing and culminating in patient usage across diverse clinical environments. Upstream analysis focuses heavily on the procurement of critical components, including microprocessors for pump control units, durable plastics for housing, and specialized textile materials for the compression sleeves and garments. Key raw material suppliers provide specialized materials that must meet stringent biocompatibility and durability standards, ensuring device safety and longevity. Research and development activities, which are highly crucial, fall within this upstream phase, focusing on miniaturization, battery technology, and algorithm development for optimized compression profiles. Manufacturing processes involve precision assembly, quality control, and rigorous regulatory adherence (such as FDA and CE Mark certifications) before the devices are ready for market distribution.
The midstream section is dominated by the complex distribution network that moves the finished DVT pumps and the consistently needed single-use garments from manufacturers to end-users. Distribution channels are typically dual: direct and indirect. Direct sales are often utilized by major established players targeting large hospital networks or Group Purchasing Organizations (GPOs), allowing for tighter control over pricing and service agreements. Indirect distribution relies on specialized medical device distributors and wholesalers who possess deep regional market knowledge and logistical expertise, especially crucial for reaching smaller clinics and home care providers. The efficiency of this distribution network is critical, particularly for ensuring the timely supply of disposable garments, which constitute a significant recurring revenue stream and must be stocked consistently to support continuous patient care.
Downstream analysis centers on the deployment, utilization, and service aspects within the end-user segments. In hospitals, the clinical decision-makers (surgeons, intensivists, nursing staff) select and implement the devices, often guided by institutional protocols. The lifecycle management includes inventory tracking, periodic maintenance, and training for clinical personnel. In the rapidly expanding home care segment, downstream activities involve rental services, patient education on proper usage, and ensuring reliable technical support, which is often outsourced or managed through specialized home medical equipment (HME) providers. The overall success of the downstream activities depends heavily on ease of use, compliance rates, and the robustness of post-sales technical support provided by manufacturers or their distributors.
Medical DVT Pumps Market Potential Customers
The primary target demographic and end-users for Medical DVT Pumps are institutional healthcare providers who manage patients at high risk of venous thromboembolism (VTE). Hospitals, particularly those with high throughput in orthopedic, neurological, trauma, and general surgery departments, represent the most significant buyer segment due to established protocols mandating mechanical prophylaxis for nearly all immobile post-operative patients. These institutions prioritize devices that offer high clinical efficacy, compatibility with existing monitoring systems, and cost-effectiveness through bulk purchasing contracts and durable pump unit construction, alongside the reliable supply of disposable garments.
Beyond traditional hospital settings, Ambulatory Surgical Centers (ASCs) constitute a rapidly growing customer base. As complex surgeries increasingly shift from inpatient to outpatient settings, ASCs require portable, easy-to-use DVT prevention solutions to maintain patient safety standards during the critical immediate post-operative phase before patients return home. Furthermore, long-term care facilities and skilled nursing facilities, housing large populations of elderly and chronically immobile individuals, are increasingly adopting DVT pumps as a preventative measure to reduce complications associated with prolonged bed rest, driven both by clinical need and efforts to reduce liability exposure.
Finally, individual consumers and home care agencies represent the fastest-expanding potential customer group. This segment requires devices that are highly intuitive, lightweight, and suitable for unsupervised use. Procurement decisions here are often influenced by physician prescriptions, reimbursement availability through Medicare or private insurance, and recommendations from home health nurses. The shift towards value-based care models and a focus on reducing hospital readmissions strongly incentivizes the use of DVT pumps in the patient's home, making this group increasingly critical for future market revenue generation.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 452.5 Million |
| Market Forecast in 2033 | USD 780.9 Million |
| Growth Rate | 7.8% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Medtronic, Zimmer Biomet, DJO Global (Enovis), ArjoHuntleigh, Cardinal Health, Breg, Devon Medical Products, Stryker Corporation, Tactical Medical, Bio Compression Systems, Normatec, ThermoTek, Flowaid Medical, Summit Medical, Mego Afek. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Medical DVT Pumps Market Key Technology Landscape
The technology landscape within the Medical DVT Pumps market is focused intensely on improving device portability, enhancing patient comfort, and integrating smart features for better clinical management. The dominant technology, Intermittent Pneumatic Compression (IPC), has seen iterative improvements in pressure gradient control, moving beyond simple sequential compression to physiologically optimized, rapid impulse inflation tailored to maximize venous flow velocity. Modern devices utilize advanced microprocessor control units that ensure precise, calibrated pressure application, often incorporating acoustic and pressure sensors to detect potential blockages or leaks, thereby maintaining consistent therapeutic delivery.
A significant technological shift involves the transition from traditional, bulky, tethered pumps to compact, battery-operated, and often tubeless devices. This miniaturization is critical for facilitating mobility in hospitalized patients and enabling effective use in the home care setting. Furthermore, disposable, single-patient use garments represent a crucial technological innovation aimed at infection control and streamlined workflow. These garments are designed for superior anatomical fit and comfort, often incorporating non-woven materials that are breathable yet robust enough to handle high-pressure cycles, thus addressing historical issues related to skin irritation and heat buildup.
The integration of Information and Communication Technology (ICT) and the Internet of Medical Things (IoMT) marks the cutting edge of DVT pump technology. New generation devices are equipped with wireless connectivity (Bluetooth or Wi-Fi) to log compliance data, operational hours, and error codes. This data is transmitted to centralized monitoring platforms or integrated directly into hospital EHRs, allowing clinicians to remotely monitor adherence and intervene promptly if prophylaxis is inadequate. This data-driven approach not only validates device usage for reimbursement purposes but also enhances overall patient safety through predictive failure alerts and adherence trend analysis, fundamentally transforming how mechanical prophylaxis is managed.
Regional Highlights
- North America: North America, particularly the United States, commands the largest market share owing to its sophisticated healthcare ecosystem and stringent clinical practice guidelines mandating DVT prophylaxis in high-risk patients. High rates of complex orthopedic surgeries, coupled with favorable reimbursement policies and high per capita healthcare expenditure, create a robust market environment. Early adoption of advanced, technologically integrated DVT pumps, especially those with IoMT capabilities for remote monitoring, ensures this region remains a key driver for global innovation and revenue.
- Europe: The European market demonstrates steady growth, driven by universal healthcare systems and a high awareness of VTE risk across clinical specialties. Germany, the UK, and France are key contributors, characterized by established clinical pathways and a strong emphasis on evidence-based prevention strategies. Regulatory bodies like the European Medicines Agency (EMA) and national health services actively promote the use of mechanical prophylaxis, supporting stable market expansion. The region is seeing increasing preference for durable, high-quality compression garments and systems suitable for long-term care settings.
- Asia Pacific (APAC): APAC is projected to exhibit the highest Compound Annual Growth Rate (CAGR) due to rapid improvements in healthcare infrastructure, increasing penetration of Western surgical standards, and the enormous, growing patient pool resulting from aging demographics and lifestyle diseases. Countries like China, India, Japan, and South Korea are key growth engines. While cost sensitivity remains a factor, increasing governmental expenditure on healthcare and rising demand for non-pharmacological VTE solutions are opening significant market opportunities for both international and local manufacturers.
- Latin America (LATAM): The LATAM market is growing moderately, primarily fueled by expanding private healthcare sectors and increasing access to advanced medical devices in major economies such as Brazil and Mexico. Market growth is often challenged by fragmented healthcare systems and variable reimbursement structures, necessitating localized marketing strategies focused on device durability and cost-effectiveness. However, increasing standardization of post-operative care procedures presents a growing demand for reliable DVT prophylaxis equipment.
- Middle East and Africa (MEA): The MEA region is characterized by disparate market conditions. The Gulf Cooperation Council (GCC) countries exhibit high adoption rates, supported by significant public investment in world-class medical facilities and high surgical volumes driven by medical tourism. In contrast, the African continent experiences slower growth, largely restricted by infrastructural deficits and affordability issues. However, targeted aid and healthcare modernization projects focusing on acute care are gradually expanding the addressable market for essential medical devices like DVT pumps.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Medical DVT Pumps Market.- Medtronic plc
- Zimmer Biomet Holdings, Inc.
- DJO Global (Enovis Corporation)
- ArjoHuntleigh AB
- Cardinal Health, Inc.
- Breg, Inc.
- Devon Medical Products
- Stryker Corporation
- Tactical Medical Corporation
- Bio Compression Systems, Inc.
- Normatec (Hyperice)
- ThermoTek, Inc.
- Flowaid Medical Systems
- Summit Medical, Inc.
- Mego Afek International
- Covidien (a Medtronic company)
- Acelity (3M)
- Haple Medical
- Air Compression Systems
- Lohmann & Rauscher
Frequently Asked Questions
Analyze common user questions about the Medical DVT Pumps market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary function and mechanism of a Medical DVT Pump?
The primary function of a Medical DVT Pump is to prevent Deep Vein Thrombosis (DVT) and resulting Pulmonary Embolism (PE) by enhancing venous blood flow in immobile patients. These pumps utilize Intermittent Pneumatic Compression (IPC) technology, applying cyclical inflation and deflation to sleeves wrapped around the limbs, mimicking natural muscle contractions to prevent blood stasis.
Are disposable compression garments a major cost driver in the DVT Pumps market?
Yes, disposable compression garments are a significant recurring operational cost. While the pump unit is reusable, the single-use garments are essential for hygiene and infection control, representing a substantial, ongoing expense for hospitals and contributing significantly to the overall total cost of ownership.
How is technological innovation driving the market, specifically regarding home care use?
Technological innovation is focused on miniaturization, creating portable, battery-operated, and tubeless DVT pumps. These advancements, coupled with wireless data logging (IoMT capabilities), facilitate ease of use, improve patient compliance outside of institutional settings, and accelerate the adoption of DVT prophylaxis in the rapidly growing home care segment.
Which geographical region holds the largest market share for DVT pumps?
North America currently holds the largest market share. This dominance is attributed to high expenditure on healthcare infrastructure, high volumes of major surgical procedures, well-established mandatory clinical guidelines for DVT prophylaxis, and strong reimbursement structures for advanced medical devices.
What are the main alternatives to DVT Pumps for VTE prevention?
The main alternatives to DVT Pumps (mechanical prophylaxis) are pharmacological agents, primarily anticoagulants such as low molecular weight heparin (LMWH) and direct oral anticoagulants (DOACs). DVT pumps are often preferred when patients have contraindications for using blood thinners due to high bleeding risk.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager