Medical Grade Chitosan Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 432132 | Date : Dec, 2025 | Pages : 257 | Region : Global | Publisher : MRU
Medical Grade Chitosan Market Size
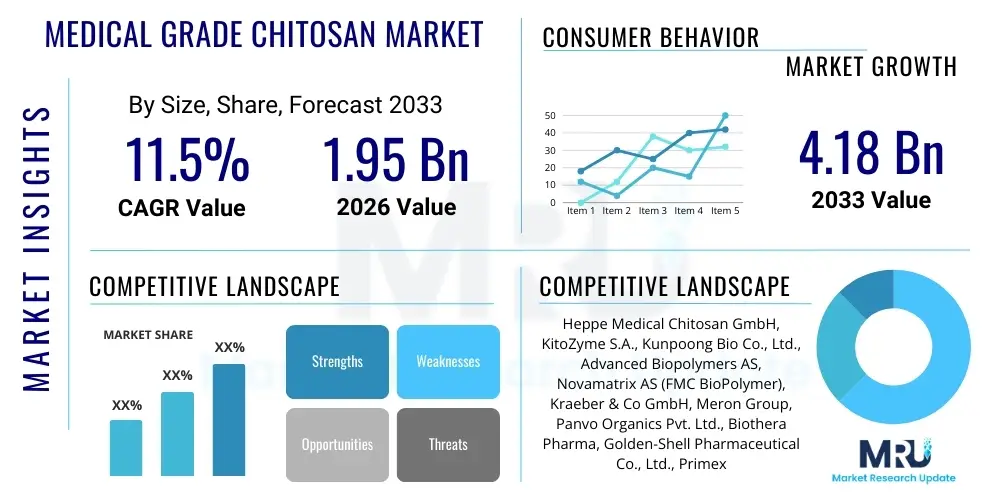
The Medical Grade Chitosan Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 11.5% between 2026 and 2033. The market is estimated at $1.95 Billion in 2026 and is projected to reach $4.18 Billion by the end of the forecast period in 2033.
Medical Grade Chitosan Market introduction
The Medical Grade Chitosan Market encompasses the production and utilization of highly purified chitosan derivatives specifically tailored for biomedical and pharmaceutical applications. Chitosan, a linear polysaccharide derived from the deacetylation of chitin—a primary component of crustacean exoskeletons—is highly valued in the medical sector due to its exceptional biocompatibility, biodegradability, non-toxicity, and unique inherent properties, including hemostatic and antimicrobial activity. The rigorous purification processes and strict quality control measures distinguish medical grade chitosan from industrial variants, ensuring suitability for direct contact with human tissues, particularly in sensitive applications like drug delivery systems and surgical interventions. This specialized biopolymer is increasingly integral to advanced medical device manufacturing, acting as a crucial enabling material for next-generation therapies and diagnostics.
Major applications of medical grade chitosan span a wide range of clinical fields, including sophisticated wound care management, where its hemostatic capability accelerates clotting and its antimicrobial nature prevents infection in chronic and acute wounds. Furthermore, it is extensively used in drug delivery as a excipient or encapsulating agent, exploiting its positive charge to bind to negatively charged cell membranes or drugs, thereby enhancing bioavailability and targeted release profiles. In regenerative medicine, chitosan scaffolds and hydrogels are foundational components for tissue engineering, providing a biodegradable matrix that supports cell proliferation and differentiation for applications such as bone repair, cartilage regeneration, and dermal substitutes. The versatility and low immunogenicity profile of this polysaccharide underpin its growing importance across surgical, therapeutic, and diagnostic domains.
The primary driving factors propelling the growth of this market include the escalating global incidence of chronic diseases, notably diabetes and cardiovascular conditions, which necessitate advanced wound dressings and complex drug administration methods. Technological advancements in biotechnology and polymer science have also enabled the development of novel chitosan derivatives with improved solubility and tailored functionality, opening up new application avenues such as injectable hydrogels and smart biomaterials. Moreover, the increasing geriatric population worldwide, prone to age-related injuries and requiring frequent surgical procedures, amplifies the demand for high-performance, biocompatible materials like medical grade chitosan. Regulatory approvals of chitosan-based medical devices and pharmaceuticals further validate its clinical efficacy and expand its market penetration.
Medical Grade Chitosan Market Executive Summary
The Medical Grade Chitosan Market is characterized by robust growth driven primarily by technological innovation in biopolymer functionalization and surging demand from the advanced wound care sector, particularly in developed economies. Business trends indicate a strong focus on strategic mergers, acquisitions, and collaborative research agreements between chitosan manufacturers and pharmaceutical companies to secure specialized supply chains and accelerate product development, especially in targeted drug delivery systems. Key manufacturers are investing heavily in optimizing the deacetylation process and functionalization techniques (e.g., quaternization, carboxymethylation) to enhance chitosan's specific properties, such as increased solubility at physiological pH, crucial for injectable formulations. Furthermore, sustainability in sourcing raw materials and ensuring batch-to-batch consistency remains a critical competitive differentiator and business imperative across the value chain.
Regionally, North America and Europe currently dominate the market share, largely due to established healthcare infrastructure, high healthcare expenditure, and a strong presence of major pharmaceutical and medical device companies actively engaged in chitosan research and product commercialization. However, the Asia Pacific (APAC) region is projected to exhibit the fastest growth trajectory during the forecast period. This accelerated expansion is attributed to rapidly improving healthcare access, increasing disposable income leading to higher demand for advanced treatments, and significant government investments in developing local biopolymer manufacturing capabilities and regenerative medicine research hubs. Specifically, countries like China, India, and South Korea are becoming pivotal centers for both consumption and low-cost, high-volume production of medical grade raw materials.
Segmentation trends highlight the increasing dominance of the Chitosan Salts and Derivatives segment, driven by the need for enhanced functionality and tailored release kinetics in sophisticated drug delivery matrices. By application, the Wound Care Management segment holds the largest market share, sustained by the high prevalence of chronic wounds, particularly diabetic ulcers, which require bio-active dressings that promote healing and prevent secondary infections. Conversely, the Tissue Engineering and Regenerative Medicine segment is anticipated to witness the highest CAGR, spurred by breakthroughs in 3D bioprinting and scaffold design utilizing chitosan hydrogels. The overall trend demonstrates a shift toward high-purity, low-molecular-weight chitosan variants that offer superior performance in complex clinical settings, demanding stringent quality specifications from suppliers.
AI Impact Analysis on Medical Grade Chitosan Market
User inquiries regarding the impact of Artificial Intelligence (AI) on the Medical Grade Chitosan Market center predominantly on themes related to process optimization, material discovery, and personalized medicine applications. Users frequently ask how AI can improve the notoriously complex and variable upstream processing of chitosan, particularly optimizing the deacetylation degree and molecular weight determination for consistent medical application quality. There is significant interest in AI's role in accelerating the discovery of novel chitosan derivatives with enhanced therapeutic efficacy, predicting their interaction with biological targets, and modeling drug release profiles within complex physiological systems. Furthermore, users anticipate AI-driven diagnostics to refine patient selection for chitosan-based therapies, leading to highly personalized and effective treatment regimens, raising expectations for reduced R&D costs and faster time-to-market for specialized chitosan products.
AI's initial application is primarily focused on optimizing the manufacturing chain. Machine learning algorithms are being deployed to analyze sensor data collected during the extraction and purification phases, allowing manufacturers to precisely control parameters like temperature, alkali concentration, and reaction time. This level of algorithmic control minimizes batch-to-batch variability, which is paramount for medical devices and pharmaceuticals, thereby improving overall yield and maintaining the stringent specifications required for FDA or EMA approval. Predictive maintenance facilitated by AI also ensures uninterrupted production and reduces waste, contributing to lower operational costs and enhanced supply chain reliability for end-user manufacturers.
In the research and development pipeline, AI and computational chemistry are revolutionizing material science related to chitosan. Deep learning models can rapidly screen thousands of potential chemical modifications (functionalization) of the chitosan backbone, predicting the resultant material's biocompatibility, solubility, and therapeutic efficacy before costly laboratory synthesis is undertaken. This 'in silico' approach accelerates the creation of novel chitosan hydrogels optimized for specific tissue engineering applications or drug carriers designed for specific oncological targets. The ability of AI to model complex biological interactions, such as cell adhesion or immune response to chitosan scaffolds, significantly reduces the experimental phase and enhances the probability of successful clinical translation.
- AI optimizes manufacturing parameters (deacetylation degree, molecular weight) to ensure batch consistency and high medical purity.
- Machine learning accelerates the discovery of novel functionalized chitosan derivatives with improved solubility and targeted delivery capabilities.
- Predictive modeling uses AI to simulate drug release kinetics from chitosan matrices, optimizing dosage forms for specific therapeutic outcomes.
- AI aids in personalized medicine by analyzing patient data to determine the optimal type and concentration of chitosan-based therapies (e.g., wound dressings).
- Computational tools enhance quality control and reduce regulatory compliance time by predicting material performance characteristics in complex biological environments.
DRO & Impact Forces Of Medical Grade Chitosan Market
The market dynamics of Medical Grade Chitosan are heavily influenced by a balanced interplay of robust drivers, significant constraints, and emerging opportunities, collectively defining the market's trajectory and competitive intensity. Primary drivers include the global expansion of the advanced wound care market, necessitated by the increasing prevalence of diabetes leading to chronic ulcers, and the material's recognized effectiveness as a superior hemostatic agent in surgical and battlefield medicine. Simultaneously, the persistent demand for materials with low toxicity and high biocompatibility in complex biomedical applications, such as internal drug delivery and surgical implants, strongly favors the adoption of natural polymers like purified chitosan. This underlying demand is further amplified by technological leaps in biomaterial processing that allow for tailored properties, making chitosan amenable to sophisticated technologies like 3D bioprinting and microencapsulation.
However, the market faces considerable restraints that temper its explosive growth potential. A critical challenge is the inherent variability and limited supply of high-purity raw chitin, primarily sourced from crustacean waste. Ensuring consistent batch-to-batch quality of medical grade chitosan—specifically in terms of degree of deacetylation and molecular weight—remains technically demanding and costly, hindering standardization across the industry. Furthermore, complex and stringent regulatory pathways, particularly in regions like North America and Europe, require extensive and expensive clinical data to approve novel chitosan-based devices or drugs, imposing significant financial burdens on smaller innovators and delaying commercialization. Consumer perception regarding materials derived from shellfish, despite rigorous purification, can occasionally present a minor marketing hurdle in certain demographic segments.
Opportunities for market growth lie predominantly in the realm of advanced material science and niche therapeutic applications. The development of water-soluble chitosan derivatives (e.g., carboxymethyl chitosan) is creating new possibilities for injectable hydrogels and ocular drug delivery, addressing a major solubility limitation of the native polymer. Furthermore, significant potential exists in leveraging chitosan's unique properties in gene therapy and cellular reprogramming, where its ability to facilitate cell penetration can be utilized for non-viral delivery systems. Strategic market penetration into emerging economies, particularly those investing heavily in modernizing their healthcare infrastructure and seeking cost-effective advanced biomaterials, represents a key growth opportunity. The market's impact forces are consequently high, driven by the indispensable role chitosan plays in modern medical interventions, especially within high-value segments like orthopedics and sophisticated pharmaceutical formulation, reinforcing its long-term viability despite sourcing challenges.
Segmentation Analysis
The Medical Grade Chitosan Market is comprehensively segmented based on its structural form (Type), intended therapeutic application (Application), and the source material from which it is derived (Source). This multi-faceted segmentation allows for a detailed analysis of market dynamics, revealing which specific derivatives or application areas are driving growth and commanding premium pricing. The purity level and chemical modification are critical determinants of market segment penetration, with high-purity, functionalized chitosan commanding the largest value share due to its requirement in sensitive implantable devices and advanced drug carriers. Understanding these segment dynamics is essential for market players to focus their research and manufacturing investments toward high-growth, high-margin sectors.
- By Type:
- Chitosan Flakes/Powder
- Chitosan Solutions
- Chitosan Gels and Hydrogels
- Chitosan Nanoparticles and Microparticles
- Chitosan Salts and Derivatives (e.g., Carboxymethyl Chitosan, Quaternized Chitosan)
- By Application:
- Wound Care Management (Dressings, Sponges)
- Drug Delivery Systems (Oral, Parenteral, Topical, Ocular)
- Tissue Engineering and Regenerative Medicine (Scaffolds, Implants)
- Surgical Sutures and Anti-adhesion Barriers
- Cosmeceuticals and Dermatology
- Medical Device Coatings
- By Source:
- Shrimp
- Crab
- Fungi/Fungal Biomass (Non-Animal Source)
Value Chain Analysis For Medical Grade Chitosan Market
The value chain for Medical Grade Chitosan is intricate and highly sequential, commencing with the raw material sourcing and culminating in the highly regulated delivery to end-user pharmaceutical and medical device manufacturers. The upstream segment is dominated by the harvesting of crustacean shells (primarily shrimp and crab) from the seafood processing industry, followed by the rigorous chemical conversion process of chitin into chitosan via deacetylation. Key challenges in the upstream stage involve ensuring a consistent, large-volume supply of high-quality chitin precursors and managing the significant environmental impact associated with chemical processing. Companies that control proprietary sourcing and highly efficient deacetylation technologies gain substantial cost and quality advantages, as the purity of the raw chitosan determines its eligibility for medical applications.
The midstream involves specialized processing, purification, and modification steps crucial for converting standard chitosan into medical grade material. This stage is characterized by high technological barriers, requiring specialized infrastructure to achieve molecular weight fractionation, degree of deacetylation precise control, sterilization, and subsequent functionalization (e.g., creating soluble derivatives or nanoparticles). Manufacturers in this segment, often high-tech chemical companies or biopolymer specialists, are responsible for tailoring the polymer's properties—such as solubility, charge density, and degradation rate—to meet the exacting specifications of diverse medical applications. Certification and regulatory adherence are paramount at this stage, adding significantly to the production cost.
The downstream segment focuses on the integration of medical grade chitosan into final product forms, primarily through distribution channels targeting pharmaceutical, wound care, and regenerative medicine industries. Direct distribution often occurs when large medical device companies purchase bulk, customized chitosan powder for incorporation into their proprietary products (e.g., composite dressings, surgical implants). Indirect channels involve specialized distributors who handle smaller volumes or niche chitosan derivatives, supplying compounding pharmacies, research institutions, and smaller biotech startups. The critical success factor in the downstream lies in establishing robust supply agreements, providing comprehensive technical support regarding formulation compatibility, and navigating regional healthcare procurement complexities, emphasizing quality assurance and traceability throughout the distribution network.
Medical Grade Chitosan Market Potential Customers
The primary consumers of Medical Grade Chitosan are highly specialized organizations within the healthcare ecosystem that leverage its unique biological and physicochemical properties for patient treatment and diagnosis. These potential customers primarily include established Pharmaceutical and Biotechnology Companies that utilize chitosan as a superior excipient for drug encapsulation, controlled release mechanisms, and as a non-viral vector in advanced gene therapy research. Their purchasing decisions are driven by purity levels, the consistency of molecular weight distribution, and the supplier's ability to provide scalability and meet stringent Good Manufacturing Practices (GMP) standards required for drug formulation approval.
A second major customer segment comprises Advanced Wound Care and Medical Device Manufacturers. These entities integrate chitosan directly into high-value products such as hemostatic bandages, transparent film dressings, anti-adhesion barriers used in surgery, and specialized materials for burn treatment. For these buyers, the key purchasing criteria revolve around the material's demonstrated efficacy in clinical trials (e.g., rapid hemostasis, enhanced healing rates), sterilization compatibility, and certification of the end-product components. Suppliers who can offer chitosan pre-formulated into hydrogels or films, reducing the customer's manufacturing complexity, often secure long-term, high-volume contracts in this demanding sector.
Furthermore, Research Institutions, Academic Centers, and Tissue Engineering Startups represent a significant, albeit typically lower volume, customer base. These organizations require highly specialized, research-grade derivatives of chitosan for developing next-generation implants, synthetic matrices, and 3D bioprinting inks aimed at regenerative medicine applications, including nerve regeneration and orthopedic repair. Their demand is characterized by a need for diverse, often customized, derivatives (e.g., ultra-low molecular weight or specific functional groups) and detailed technical documentation supporting experimental use. As R&D progresses and these experimental products move toward commercialization, these customers rapidly transform into high-volume purchasers, representing significant future growth potential for core chitosan suppliers.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | $1.95 Billion |
| Market Forecast in 2033 | $4.18 Billion |
| Growth Rate | 11.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Heppe Medical Chitosan GmbH, KitoZyme S.A., Kunpoong Bio Co., Ltd., Advanced Biopolymers AS, Novamatrix AS (FMC BioPolymer), Kraeber & Co GmbH, Meron Group, Panvo Organics Pvt. Ltd., Biothera Pharma, Golden-Shell Pharmaceutical Co., Ltd., Primex Ehf, G.T.C. Bio Corp., Dalian Xindongyuan Biologic Technology Co., Ltd., Xi'an Rainbow Biotech Co., Ltd., Amsal Chem. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Medical Grade Chitosan Market Key Technology Landscape
The technological landscape of the Medical Grade Chitosan Market is dominated by advanced purification, precise chemical modification, and novel formulation techniques designed to enhance the biopolymer's functional properties for clinical efficacy. A core technological focus is the optimization of the deacetylation process (the conversion of chitin to chitosan) using highly controlled methods, such as microwave-assisted or enzymatic hydrolysis, which allow for the precise tuning of the degree of deacetylation (DDA) and molecular weight (MW). Precision in DDA and MW is crucial, as these characteristics directly influence the material's solubility, biodegradability, and biological activity (e.g., antimicrobial strength, mucoadhesiveness). Companies deploying proprietary fractional purification techniques to achieve ultra-high purity levels and narrow molecular weight ranges gain a significant competitive edge in supplying demanding application segments like implantable devices and ocular drug delivery systems.
Beyond basic synthesis, the development of functionalized chitosan derivatives represents a major technological advancement. Technologies such as quaternization, PEGylation, and carboxymethylation are employed to chemically modify the chitosan structure, primarily to overcome its major limitation: poor solubility at physiological pH (around 7.4). For instance, quaternized chitosan exhibits enhanced water solubility and superior antimicrobial properties, making it ideal for use in liquid formulations or coatings. Similarly, the integration of chitosan into nanotechnology involves advanced techniques like ionic gelation, emulsification solvent diffusion, and microfluidics for the controlled production of chitosan nanoparticles and microparticles. These nanoscale carriers are essential for targeted drug delivery, protecting sensitive biological drugs and ensuring precise release kinetics at diseased sites, thereby significantly improving therapeutic indices.
Furthermore, formulation technologies are rapidly evolving, particularly in the realm of 3D bioprinting and injectable hydrogels. Chitosan, often blended with other polymers like alginate or gelatin, forms the basis of bio-inks. Key technological challenges include optimizing the rheological properties of these chitosan-based bio-inks to ensure printability while maintaining cell viability and structural integrity post-printing. Similarly, thermosensitive or pH-responsive injectable hydrogels utilize specially modified chitosan to transition from a liquid to a stable gel state upon introduction into the body. These advanced materials offer minimally invasive solutions for tissue filling, localized drug release, and cell transplantation, underscoring the shift toward high-tech, minimally invasive application methodologies that define the modern medical grade chitosan market.
Regional Highlights
- North America: Market Leader Due to R&D and High Healthcare Spending
- Europe: Focus on Advanced Dressings and Regulatory Compliance
- Asia Pacific (APAC): Fastest Growing Market with Expanding Healthcare Infrastructure
- Latin America (LATAM) and Middle East & Africa (MEA): Emerging Markets
North America, encompassing the United States and Canada, maintains the largest share of the Medical Grade Chitosan Market, primarily driven by substantial investments in regenerative medicine, a highly developed pharmaceutical sector, and robust adoption of advanced wound care products. The U.S. acts as the global hub for innovation, with numerous biotechnology startups and large medical device manufacturers leveraging high-purity chitosan for clinical applications, especially in orthopedic surgery and complex drug delivery systems. High healthcare expenditure facilitates rapid adoption of premium, cutting-edge chitosan-based medical technologies. Furthermore, strict but clear regulatory frameworks, such as those established by the FDA, foster confidence among manufacturers, encouraging the development and rapid commercialization of chitosan derivatives tailored for internal use and surgical procedures. The region's aging population further fuels demand for materials with superior hemostatic and tissue repair capabilities.
The North American market is also characterized by strong academic-industry collaboration focused on overcoming formulation challenges related to chitosan solubility and stability. Key R&D efforts center on creating injectable chitosan hydrogels for minimally invasive procedures and utilizing chitosan nanoparticles for targeted cancer drug delivery. Competition is intense, focusing on product differentiation based on purity, molecular weight consistency, and compliance with stringent biomaterial standards. The high concentration of large clinical research organizations (CROs) also accelerates the time taken for chitosan products to move from bench to bedside, solidifying the region's dominant market position.
Europe represents the second-largest market, characterized by stringent regulatory environments (EMA, MDR) that emphasize material safety and quality, particularly in Germany, the UK, and France. The European market exhibits strong demand for advanced antimicrobial wound dressings and implantable devices utilizing chitosan. Countries like Germany are major production centers for high-quality chitosan derivatives, focusing heavily on sustainable sourcing and environmentally conscious manufacturing processes. The market growth here is underpinned by established public health systems that readily integrate cost-effective yet high-performance wound management solutions to address rising diabetes-related morbidities.
Innovation in Europe leans toward applications in dermatological treatments and controlled drug release for chronic conditions. Regulatory standards necessitate extensive documentation regarding the polymer's origin and processing, favoring established suppliers who can guarantee purity and traceability. Furthermore, the push towards biodegradable and bio-sourced materials across the EU aligns perfectly with the inherent properties of chitosan, fostering its greater utilization in environmentally sensitive medical applications and cosmetic products. This stringent regulatory landscape ensures high barriers to entry but guarantees premium quality products for consumers.
The APAC region is projected to register the highest CAGR during the forecast period, driven by rapid improvements in healthcare infrastructure, increasing awareness regarding advanced medical treatments, and massive government spending on public health initiatives. Countries like China, Japan, India, and South Korea are key growth engines. China and India, in particular, benefit from vast seafood processing industries, providing local manufacturers with readily available chitin raw materials, leading to cost-competitive production of chitosan. While historical focus was on bulk commodity chitosan, there is a distinct shift toward high-purity medical grade production to meet international standards.
Growth in APAC is significantly influenced by the expanding geriatric population in nations like Japan and South Korea, which increases the demand for orthopedic and regenerative medicine solutions where chitosan scaffolds are highly applicable. Furthermore, the region is becoming a global manufacturing hub for generic drugs and medical disposables, driving bulk demand for chitosan excipients and materials for medical device coatings. Regional players are increasingly investing in R&D, often in collaboration with Western entities, to quickly adopt functionalization technologies and penetrate the high-value segments of advanced wound care and novel drug delivery, signaling strong market maturation and future dominance.
LATAM and MEA represent emerging markets with nascent but accelerating adoption rates. Growth in these regions is primarily spurred by increasing access to modern surgical techniques and rising foreign direct investment in healthcare facilities. While market penetration is currently lower due to infrastructural limitations and reliance on imports for specialized derivatives, the local demand for basic hemostatic agents and cost-effective wound dressings is strong. Regional opportunities focus on utilizing local raw material sources (where applicable) and developing foundational chitosan products for basic trauma and infection control in developing healthcare systems. Future growth will be contingent on economic stability and increased regulatory harmonization facilitating easier market access for international medical grade products.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Medical Grade Chitosan Market.- Heppe Medical Chitosan GmbH
- KitoZyme S.A.
- Kunpoong Bio Co., Ltd.
- Advanced Biopolymers AS
- Novamatrix AS (FMC BioPolymer)
- Kraeber & Co GmbH
- Meron Group
- Panvo Organics Pvt. Ltd.
- Biothera Pharma
- Golden-Shell Pharmaceutical Co., Ltd.
- Primex Ehf
- G.T.C. Bio Corp.
- Dalian Xindongyuan Biologic Technology Co., Ltd.
- Xi'an Rainbow Biotech Co., Ltd.
- Amsal Chem
- Foodchem International Corporation
- Biophrame Technologies
- Marshall Marine Products
- Jefo Group
Frequently Asked Questions
Analyze common user questions about the Medical Grade Chitosan market and generate a concise list of summarized FAQs reflecting key topics and concerns.What are the primary medical uses of high-purity chitosan?
High-purity medical grade chitosan is primarily utilized in advanced wound care management for its superior hemostatic and antimicrobial properties, accelerating wound healing. It is also crucial in drug delivery systems as an encapsulating agent for controlled release, and extensively used in regenerative medicine for creating biocompatible scaffolds for tissue engineering.
How is "Medical Grade" Chitosan differentiated from standard industrial grade chitosan?
Medical Grade Chitosan is differentiated by its significantly higher purity, strict control over the degree of deacetylation (DDA) and molecular weight (MW) distribution, and minimal heavy metal/protein residues. It must adhere to stringent quality standards (e.g., GMP) and be biocompatible, sterile, and often endotoxin-free, making it suitable for direct clinical application, unlike standard industrial grades.
What challenges exist regarding the sourcing and consistency of medical grade chitosan?
The main challenges involve sourcing consistent, large-volume supplies of high-quality chitin precursor, primarily derived from crustacean shells, which leads to raw material price volatility. Achieving batch-to-batch consistency in crucial properties (DDA and MW) during the purification process is technically demanding and costly, which limits standardization across the market.
Which geographical region exhibits the fastest growth potential for medical grade chitosan applications?
The Asia Pacific (APAC) region is projected to demonstrate the fastest Compound Annual Growth Rate (CAGR). This acceleration is fueled by rapidly expanding healthcare infrastructure, significant government investments in regenerative medicine, and increasing adoption of advanced medical treatments in densely populated economies like China and India.
How do novel chitosan derivatives enhance drug delivery capabilities?
Novel chitosan derivatives, such as carboxymethyl or quaternized chitosan, enhance drug delivery by improving the polymer’s solubility at physiological pH, enabling the creation of stable injectable hydrogels and highly functional nanoparticles. These modifications allow for targeted drug delivery, improved bioavailability, and the precise control of release kinetics, crucial for complex therapeutics like gene therapy vectors.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
