Microbial Air Samplers Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 433066 | Date : Dec, 2025 | Pages : 243 | Region : Global | Publisher : MRU
Microbial Air Samplers Market Size
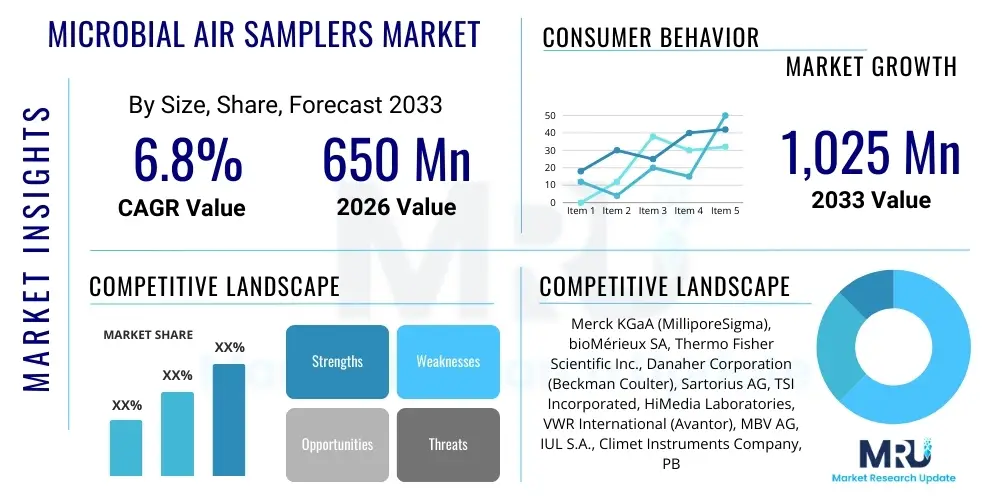
The Microbial Air Samplers Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2026 and 2033. The market is estimated at USD 650 Million in 2026 and is projected to reach USD 1,025 Million by the end of the forecast period in 2033.
Microbial Air Samplers Market introduction
Microbial air samplers are precision instruments critical for environmental monitoring, specifically designed to capture airborne viable microorganisms such as bacteria, molds, and yeasts onto a culture medium for subsequent incubation and enumeration. These devices are essential components in maintaining quality assurance and regulatory compliance across sensitive environments, particularly in sterile manufacturing facilities, healthcare settings, and research laboratories. The primary mechanism often involves impaction or filtration, ensuring accurate quantification of bioburden in controlled environments, thereby mitigating risks associated with contamination and ensuring product safety and integrity, which drives their adoption in industries governed by stringent contamination control standards.
The core product portfolio encompasses several types, including slit-to-agar samplers, centrifugal air samplers, and sieve samplers, with modern variants incorporating continuous monitoring capabilities and portability features to address diverse operational requirements. Major applications span the pharmaceutical and biotechnology sectors, where sterile processing demands absolute control over environmental bioburden, as well as the food and beverage industry for hygiene validation, and clinical settings for infection control. The inherent benefits of these samplers include high accuracy, ability to validate cleanroom classifications (e.g., ISO Class 5, 7), and provision of auditable data necessary for regulatory submissions and operational documentation, making them indispensable tools for quality control managers.
Driving factors contributing to the robust market expansion include the escalating global demand for sterile injectable drugs and biopharmaceuticals, necessitating heightened environmental monitoring compliance with organizations like the FDA, EMA, and WHO. Furthermore, the increasing complexity of manufacturing processes in advanced therapies and the growing awareness of airborne disease transmission in hospital environments are accelerating the deployment of advanced, often automated, microbial air sampling technologies. Technological advancements, particularly the integration of IoT for real-time data logging and remote diagnostics, further enhance their efficiency and reliability, propelling market growth across various end-user segments globally.
Microbial Air Samplers Market Executive Summary
The Microbial Air Samplers Market is characterized by intense focus on compliance and automation, driven primarily by the global growth of the pharmaceutical and biotechnology industries. Key business trends include the shift towards continuous and active monitoring systems over traditional periodic sampling, which offers superior data integrity and immediate identification of contamination events. Manufacturers are prioritizing the development of compact, portable, and user-friendly devices that integrate seamlessly with existing Laboratory Information Management Systems (LIMS). Mergers and acquisitions focused on expanding geographical reach and integrating advanced sensor technologies remain a pivotal strategy for market leaders aiming to consolidate their positions and offer comprehensive environmental control solutions.
Regionally, North America maintains the dominant market share, attributed to the presence of major biopharmaceutical companies, rigorous regulatory frameworks established by the FDA, and high expenditure on research and development activities focused on contamination control technologies. However, the Asia Pacific (APAC) region is poised for the fastest growth, fueled by rapid industrialization, increasing governmental focus on healthcare infrastructure development, and the expansion of generic drug manufacturing capabilities in countries like China and India, requiring stricter adherence to international cleanroom standards. Europe also represents a mature market, strongly supported by the presence of large medical device and pharmaceutical manufacturing bases adhering to EU GMP guidelines.
Segmentation trends indicate that impaction-based samplers, particularly centrifugal models offering high collection efficiency, hold the largest market share, while continuous monitoring systems are anticipated to exhibit the highest growth trajectory due to their efficiency in detecting intermittent contamination events critical in Grade A and B cleanroom areas. The pharmaceutical and biotechnology segment remains the primary application area, generating the highest revenue due to the critical nature of sterility assurance. Furthermore, there is a distinct trend towards single-use consumables and validated calibration services, which contribute significantly to the aftermarket services revenue streams for key players.
AI Impact Analysis on Microbial Air Samplers Market
Common user questions regarding AI's influence in the Microbial Air Samplers market center on how AI can automate contamination risk assessment, predict failure points in cleanroom environments, and enhance data interpretation beyond manual colony counting. Users are keen to understand the shift from reactive monitoring to proactive predictive quality control, asking if AI integration can drastically reduce false positive rates and the time taken for root cause analysis following a contamination incident. The collective expectation is that AI algorithms, leveraging vast datasets from historical sampling, temperature, pressure, and humidity readings, will move the industry toward truly smart environmental monitoring systems, enabling optimized sampling schedules and reducing operational costs associated with non-compliant batches, thereby significantly enhancing overall manufacturing efficiency and compliance posture.
- AI algorithms enable predictive maintenance of air sampler hardware, minimizing downtime and ensuring continuous measurement accuracy crucial for sterile environments.
- Advanced pattern recognition aids in identifying environmental anomalies that correlate with potential contamination risks, allowing for proactive intervention before regulatory breaches occur.
- Machine learning models integrate microbial sampling data with HVAC parameters (differential pressure, air changes per hour) to optimize cleanroom operation and energy usage while maintaining compliance.
- AI-powered image analysis automates and standardizes the interpretation of culture plate results, replacing subjective manual colony counting with objective, high-throughput analysis.
- Generative AI tools assist in drafting comprehensive compliance reports and Standard Operating Procedures (SOPs) based on observed monitoring data and regulatory updates.
DRO & Impact Forces Of Microbial Air Samplers Market
The Microbial Air Samplers Market is propelled by stringent regulatory mandates and technological integration, while facing significant challenges related to cost and complex validation processes. Drivers include the increasing global pharmaceutical manufacturing output, which requires validated aseptic environments, and heightened public health concerns necessitating efficient infection control in hospitals. Opportunities arise from the convergence of monitoring technologies, notably the shift towards real-time viable particle counting coupled with conventional culturing methods, offering robust hybrid systems. The impact forces are predominantly driven by regulatory evolution—such as updates to Annex 1 of the EU GMP guidelines—which continuously raise the standards for environmental monitoring, compelling manufacturers to invest in newer, compliant sampling technology.
Restraints primarily revolve around the high initial capital investment required for automated and continuous microbial monitoring systems, particularly for smaller manufacturing facilities or clinical laboratories with limited budgets. Furthermore, the operational complexity involved in the calibration, validation, and routine maintenance of these highly sensitive devices presents a significant hurdle, requiring specialized technical expertise. The potential for human error during manual plate handling and the inherent delay of 48-72 hours in obtaining culture-based results also limit immediate responsiveness, although newer rapid methods are attempting to mitigate this time constraint, creating a competitive pressure on traditional samplers.
The opportunity landscape is vast, particularly in integrating air sampling data into centralized smart factory platforms (Industry 4.0), allowing for holistic contamination risk management across entire production facilities. Moreover, the expanding field of environmental monitoring in non-traditional settings, such as cannabis cultivation facilities and advanced material science laboratories, presents new application avenues. The demand for portable, wireless, and IoT-enabled samplers designed for field use and remote diagnostics highlights a key area for future product development and market expansion, especially in emerging economies where centralized monitoring infrastructure is still nascent.
Segmentation Analysis
The Microbial Air Samplers market is broadly segmented based on product type, application, and end-user, reflecting the diverse needs of quality control and sterile manufacturing environments globally. Analyzing these segments helps stakeholders understand specific demand patterns, technological preferences, and growth pockets within the market. Impaction-based samplers dominate the product landscape due to their established validation protocols and compliance history, while the pharmaceutical sector drives the highest revenue due to its stringent sterility requirements and large-scale manufacturing operations worldwide.
- By Product Type: Active Air Samplers (Impaction Air Samplers, Filtration Air Samplers, Centrifugal Air Samplers), Passive Air Samplers (Settling Plates).
- By Method: Manual Air Samplers, Automated/Continuous Air Samplers.
- By Application: Pharmaceutical and Biotechnology Manufacturing, Clinical and Healthcare Settings, Food and Beverage Industry, Environmental Monitoring and Research, Cosmetics Industry.
- By End-User: Pharmaceutical and Biotechnology Companies, Hospitals and Diagnostic Laboratories, Food Testing Laboratories, Academic and Research Institutes, Environmental Agencies.
Value Chain Analysis For Microbial Air Samplers Market
The value chain for microbial air samplers is complex, beginning with highly specialized upstream suppliers and culminating in validation and support services provided downstream. Upstream analysis involves raw material sourcing, primarily high-precision components such as sterile plastics for consumables, calibrated airflow sensors, high-efficiency motors, and specialized microprocessors for data processing. Key activities at this stage include precision engineering and quality control of components to ensure consistent and accurate air collection volumes, which is crucial for compliant monitoring. The consolidation of component suppliers is a notable trend, driven by the need for validated and traceable materials that meet ISO standards for cleanroom instrumentation.
Midstream activities involve core manufacturing, assembly, and rigorous calibration of the final sampling devices. Original Equipment Manufacturers (OEMs) invest heavily in R&D to integrate features like wireless connectivity (IoT), improved battery life for portability, and user-friendly interfaces. The quality and reliability of the firmware and software for data logging are paramount, necessitating extensive software validation protocols. Downstream distribution is crucial; samplers and associated consumables (like prepared media plates) are often distributed through specialized channels—a mix of direct sales teams for major pharmaceutical accounts and authorized third-party distributors or resellers for wider geographic coverage, particularly in emerging markets.
Distribution channels are categorized into direct and indirect methods. Direct sales allow OEMs to maintain control over pricing, installation, and post-sale technical support, which is critical given the high-value nature and regulatory sensitivity of the equipment. Indirect channels, utilizing regional distributors, are essential for accessing fragmented markets and providing localized support, including certified calibration services and maintenance contracts. Potential conflicts in the channel arise concerning the intellectual property of consumables, as manufacturers aim to capture recurring revenue from proprietary media plates necessary for device validation, creating a competitive aftermarket landscape.
Microbial Air Samplers Market Potential Customers
The primary end-users and potential buyers of microbial air samplers are institutions operating highly controlled or sterile environments where airborne microbial contamination poses a critical risk to product integrity, patient safety, or operational compliance. Pharmaceutical and biotechnology companies are the largest consumer segment, including manufacturers of aseptic drugs, vaccines, biologics, and sterile medical devices, who rely on these samplers for continuous monitoring of Grade A and B cleanrooms as mandated by global regulatory bodies like the FDA and EMA. Quality assurance and quality control departments within these organizations drive the procurement decisions, focusing on validated, compliant systems capable of integration with existing environmental monitoring platforms.
Hospitals and clinics represent a significant and growing customer base, particularly for infection control measures in operating theaters, sterile processing departments (SPDs), and isolation wards. Clinical microbiologists and infection control practitioners require portable and easy-to-use samplers to rapidly assess potential contamination sources and validate cleaning and disinfection protocols, especially during outbreaks. The rising focus on Hospital-Acquired Infections (HAIs) and stricter standards for surgical suite air quality are compelling healthcare facilities to upgrade from traditional, passive methods to active sampling systems for reliable environmental data necessary for accreditation and safety audits.
Furthermore, food and beverage processing facilities, particularly those dealing with dairy, ready-to-eat meals, and brewing, require air sampling to maintain hygiene standards and prevent product spoilage due to mold or bacterial contamination. Environmental testing laboratories and academic research institutes focused on aerobiology, air quality studies, and microbiology research also constitute essential buyers. These customers prioritize high sensitivity, flexibility, and the ability to interface with various data analysis software for complex experimental monitoring and long-term regulatory reporting requirements, creating demand for sophisticated, often research-grade, instrumentation.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 650 Million |
| Market Forecast in 2033 | USD 1,025 Million |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Merck KGaA (MilliporeSigma), bioMérieux SA, Thermo Fisher Scientific Inc., Danaher Corporation (Beckman Coulter), Sartorius AG, TSI Incorporated, HiMedia Laboratories, VWR International (Avantor), MBV AG, IUL S.A., Climet Instruments Company, PBI International, R&D Systems (Biotechne), Lighthouse Worldwide Solutions, Emtek LLC, F. Hoffmann-La Roche AG, 3P Engineering, Biotest AG, Bio-Rad Laboratories, PMS Particle Measuring Systems. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Microbial Air Samplers Market Key Technology Landscape
The microbial air samplers market is undergoing a significant technological transformation, moving beyond basic impaction and filtration methods towards sophisticated, integrated monitoring platforms. A key technological driver is the maturation of real-time viable particle counters (RVPCs). These advanced systems utilize laser-induced fluorescence (LIF) technology to differentiate viable biological particles from non-viable dust particles instantly, eliminating the 48-72 hour waiting period required for traditional culture methods. This real-time data acquisition is crucial for continuous process control and immediate investigation of contamination sources in critical clean zones (ISO Grade A/B), offering unparalleled speed and responsiveness in quality assurance environments.
Another dominant trend is the integration of Internet of Things (IoT) capabilities and wireless connectivity into air sampling devices. Modern samplers are increasingly equipped with sensors and communication modules that allow them to connect seamlessly to a centralized data logging system or cloud-based platforms. This IoT integration facilitates remote monitoring, automated data collection, electronic record keeping (essential for 21 CFR Part 11 compliance), and facilitates sophisticated trend analysis. The ability to monitor environmental parameters, such as temperature and humidity, concurrently with bioburden collection enhances the contextual understanding of potential contamination events, thereby improving the robustness of environmental monitoring programs.
Automation and modularity define the latest generation of samplers, particularly those designed for high-throughput sterile manufacturing lines. Automated sampling systems reduce manual handling, thereby minimizing the risk of technician-induced contamination and improving data integrity. Furthermore, the focus on developing standardized, validated consumables, such as pre-filled, sterile media cartridges and single-use impaction heads, reduces preparation time and variability in results. The technological push is consistently towards high throughput, low intervention, and full data traceability, ensuring that monitoring systems can keep pace with the efficiency demands of modern biopharmaceutical manufacturing.
Regional Highlights
- North America: North America, particularly the United States, commands the largest market share owing to the well-established presence of major pharmaceutical and biotechnology companies and significant investments in sterile manufacturing R&D. The region is characterized by stringent regulatory environments, including adherence to Current Good Manufacturing Practices (cGMP) enforced by the FDA, which mandates advanced environmental monitoring protocols. High levels of technological adoption, coupled with substantial healthcare expenditure, drive demand for high-end automated and real-time microbial sampling systems. The continuous need for validation in advanced therapy manufacturing (ATMPs) further solidifies North America’s leading position in adopting cutting-edge monitoring solutions.
- Europe: Europe represents a mature market, holding the second-largest share, primarily driven by the robust presence of global pharmaceutical hubs in countries like Germany, Switzerland, and Ireland. The implementation of strict guidelines, notably the updated EU GMP Annex 1 concerning the manufacture of sterile medicinal products, has necessitated mandatory upgrades to air monitoring equipment, favoring continuous monitoring over traditional methods. Regulatory harmonization across the EU facilitates market entry, and strong governmental focus on patient safety and sterile drug quality ensures steady demand for certified, high-precision air sampling devices and associated consumables throughout the forecast period.
- Asia Pacific (APAC): The APAC region is projected to experience the fastest growth rate, fueled by the rapid expansion of the healthcare sector, growing biopharmaceutical production, and increasing outsourcing of manufacturing activities to countries like China, India, and South Korea. Increased foreign direct investment (FDI) in local pharmaceutical industries, combined with rising awareness and adoption of international quality standards (e.g., ISO cleanroom standards), creates substantial market opportunities. While historically price-sensitive, increasing regulatory enforcement and modernization of manufacturing facilities are driving a shift towards sophisticated, automated air samplers, particularly in key manufacturing zones.
- Latin America (LATAM): The LATAM market growth is steady, driven by infrastructure improvements in healthcare and pharmaceutical manufacturing, particularly in Brazil and Mexico. The market is often characterized by a preference for cost-effective, portable sampling solutions. Regulatory frameworks are evolving, often mirroring US and European standards, leading to increased demand for validated equipment, although capital expenditure constraints sometimes slow the adoption of fully continuous monitoring systems.
- Middle East and Africa (MEA): The MEA market is nascent but growing, primarily driven by investments in high-tech healthcare facilities and local pharmaceutical production initiatives aimed at reducing reliance on imports, particularly in the UAE and Saudi Arabia. Market penetration is generally focused on essential, reliable impaction samplers for hospitals and new cleanroom setups. Future growth hinges heavily on regulatory maturity and continued investment in high-standard manufacturing infrastructure required for quality pharmaceutical and food production.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Microbial Air Samplers Market.- Merck KGaA (MilliporeSigma)
- bioMérieux SA
- Thermo Fisher Scientific Inc.
- Danaher Corporation (Beckman Coulter)
- Sartorius AG
- TSI Incorporated
- HiMedia Laboratories
- VWR International (Avantor)
- MBV AG
- IUL S.A.
- Climet Instruments Company
- PBI International
- R&D Systems (Biotechne)
- Lighthouse Worldwide Solutions
- Emtek LLC
- F. Hoffmann-La Roche AG
- 3P Engineering
- Biotest AG
- Bio-Rad Laboratories
- PMS Particle Measuring Systems
Frequently Asked Questions
Analyze common user questions about the Microbial Air Samplers market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary difference between active and passive microbial air sampling methods?
Active microbial air sampling utilizes a mechanical device, such as an air sampler pump, to draw a measured volume of air over or through a collection medium (usually a petri dish or agar strip) over a specific time interval. This method provides quantitative data (Colony Forming Units per volume of air, CFU/m³) crucial for regulatory compliance in cleanrooms. Conversely, passive sampling involves exposing an open petri dish (settle plate) to the ambient environment for a defined period, relying solely on gravity for microbial settlement. Passive sampling provides qualitative data about potential contamination but cannot measure the concentration of microorganisms per unit volume of air, limiting its use in critical Grade A/B zones where precise quantification is mandatory.
Why are real-time viable particle counters considered a significant technological advancement in the market?
Real-time viable particle counters (RVPCs) represent a pivotal technological advancement because they eliminate the critical delay inherent in traditional culture-based methods. Traditional samplers require incubation time (typically 2-7 days) before results are available, meaning contamination events are only identified retrospectively. RVPCs use laser-induced fluorescence (LIF) to instantly detect and differentiate between viable (living) biological particles and non-viable inert particles in the air stream. This provides instantaneous feedback, enabling manufacturers in highly sensitive aseptic processing areas to immediately halt operations, identify the root cause, and prevent potentially contaminated product batches, thereby drastically improving quality control responsiveness and reducing financial losses associated with batch rejection.
How does regulatory compliance, particularly Annex 1 of the EU GMP, influence the purchasing decisions for air samplers?
The updated Annex 1 of the EU GMP guidelines places increased emphasis on contamination control strategy (CCS) and continuous monitoring, significantly influencing purchasing decisions. Specifically, Annex 1 mandates continuous or frequent monitoring of critical zones (Grade A/B) and requires equipment to be qualified and calibrated with a focus on data integrity. This drives demand away from simple, periodic manual samplers towards automated, integrated, continuous monitoring systems that can provide robust, traceable electronic records. Compliance requirements force end-users, especially in Europe, to invest in higher-quality, validated instruments with features like automated sampling plans and seamless integration with environmental monitoring system software to ensure audit readiness.
What are the key challenges associated with ensuring the accuracy and calibration of microbial air sampling devices?
Ensuring the accuracy of microbial air samplers presents several challenges, primarily centered on maintaining the precise flow rate and air volume measurement accuracy. Samplers must be calibrated annually, or more frequently if used intensively, using traceable calibration standards to guarantee the accuracy of the measured air volume. Impaction efficiency must be validated to ensure microorganisms are effectively captured without desiccation or loss of viability upon collection. Furthermore, validation complexity increases with automated systems, requiring rigorous performance qualification (PQ) and operational qualification (OQ) protocols to confirm that the device performs reliably under operational conditions, including managing variables such as filter blockage or pump wear which can subtly affect the sampling rate over time, compromising regulatory compliance.
In which application segment is the highest growth anticipated for the Microbial Air Samplers Market?
The highest anticipated growth for the Microbial Air Samplers Market is expected in the Pharmaceutical and Biotechnology application segment, particularly driven by the production of Advanced Therapy Medicinal Products (ATMPs) such as cell and gene therapies. Manufacturing these sensitive biologics demands extraordinarily high levels of environmental control and sterility assurance, pushing companies to adopt continuous, highly automated monitoring solutions. Moreover, the increasing global manufacturing capacity for vaccines and sterile injectable drugs, particularly in APAC and emerging markets, necessitates the rapid deployment of compliant microbial air sampling technologies to meet global quality standards and satisfy the expanding demand for sterile healthcare products.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
- Microbial Air Samplers Market Statistics 2025 Analysis By Application (Pharmaceutical, Food & Beverage, Scientific Laboratory), By Type (Portable Microbial Air Sampler, Desktop Microbial Air Sampler), and By Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Forecast 2025 to 2032
- Active Microbial Air Samplers Market Statistics 2025 Analysis By Application (Pharmaceuticals, Food & Beverages, Hospitals & Clinics), By Type (Portable Microbial Air Samplers, Desktop Microbial Air Samplers), and By Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Forecast 2025 to 2032
- Microbial Air Samplers Market Size, Share, Trends, & Covid-19 Impact Analysis By Type (Portable Microbial Air Sampler, Desktop Microbial Air Sampler), By Application (Pharmaceutical, Cosmetics, Food & beverage industries), By Region - North America, Latin America, Europe, Asia Pacific, Middle East, and Africa | In-depth Analysis of all factors and Forecast 2023-2030
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
