Natural Desiccated Thyroid Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 432651 | Date : Dec, 2025 | Pages : 245 | Region : Global | Publisher : MRU
Natural Desiccated Thyroid Market Size
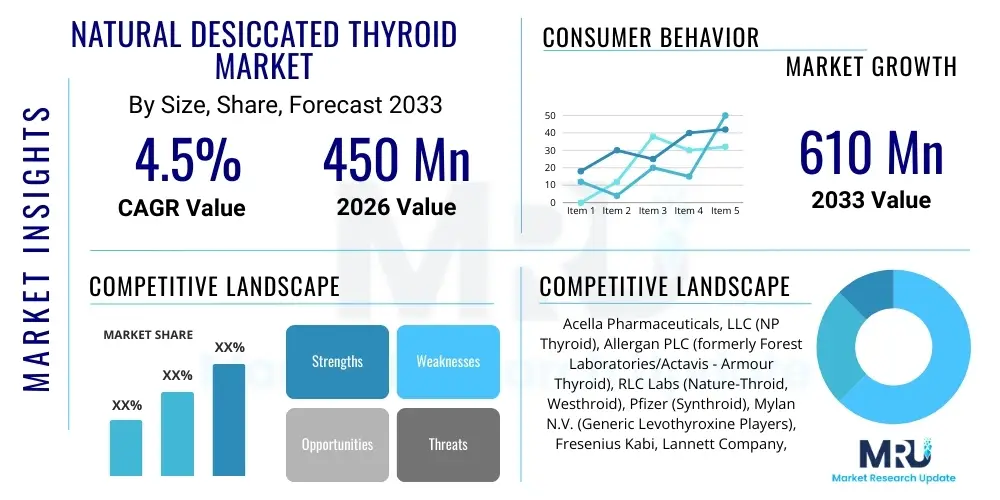
The Natural Desiccated Thyroid Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 4.5% between 2026 and 2033. The market is estimated at USD 450 Million in 2026 and is projected to reach USD 610 Million by the end of the forecast period in 2033.
Natural Desiccated Thyroid Market introduction
The Natural Desiccated Thyroid (NDT) Market comprises pharmaceutical products derived from the dried thyroid glands of pigs (porcine origin), utilized primarily for the treatment of hypothyroidism. NDT preparations contain a combination of both T4 (levothyroxine) and T3 (liothyronine), along with other thyroid co-factors, mimicking the natural hormones produced by the human thyroid gland. This distinct profile, contrasting sharply with synthetic single-hormone therapies (like levothyroxine, which is pure T4), appeals strongly to a subset of patients who report persistent symptoms despite achieving euthyroid status on synthetic medication. The product is sought after by patients with persistent symptoms such as fatigue, weight gain, and brain fog, believing that the complete profile of NDT offers superior symptomatic relief and overall well-being compared to standard synthetic options.
The key driving factors propelling the growth of this market include increasing patient awareness regarding alternative hypothyroidism treatments and the robust advocacy from patient groups and integrated medicine practitioners who champion NDT's efficacy in specific patient populations. Furthermore, the rising global prevalence of chronic thyroid disorders, catalyzed by lifestyle changes, dietary shifts, and environmental factors, continuously expands the base of potential NDT users. While NDT faces significant regulatory and manufacturing challenges related to potency standardization and sourcing variability, its continued presence in clinical practice is maintained by demonstrated patient satisfaction in cases where T4-only treatment proves inadequate for symptom resolution.
Natural Desiccated Thyroid Market Executive Summary
The Natural Desiccated Thyroid market demonstrates resilience driven by strong patient preference and the expansion of integrated and functional medicine practices, despite being a specialized niche within the broader thyroid treatment landscape dominated by synthetic agents. Key business trends indicate a concentrated market structure where established manufacturers focus heavily on improving potency standardization techniques and addressing regulatory concerns regarding consistency, leading to strategic investments in advanced quality control measures. Regional trends highlight North America, particularly the United States, as the primary consumer base due to established infrastructure supporting alternative medical approaches and high levels of patient autonomy in treatment choices. Conversely, European markets exhibit slower adoption due to stringent pharmaceutical regulations concerning animal-derived hormonal preparations, presenting both market entry hurdles and expansion opportunities should regulatory acceptance evolve.
Segment trends reveal that the capsule/tablet formulation remains the dominant segment due to ease of administration and established clinical use, though demand for compounded, customized NDT preparations is growing, driven by personalized medicine trends. The application segment is overwhelmingly dominated by primary hypothyroidism treatment, while subclinical hypothyroidism treatment represents a marginal yet growing area of interest as diagnostic methodologies improve. The primary constraint impacting overall market growth involves the inherent challenge of ensuring batch-to-batch consistency in the hormone content (T4 and T3 ratios) derived from porcine glands, which subjects the market to higher regulatory scrutiny and intermittent supply chain challenges. Successful market participants are those who navigate this regulatory complexity while maintaining strong relationships with patient advocacy groups and specialized prescribing physicians.
AI Impact Analysis on Natural Desiccated Thyroid Market
Common user questions regarding AI's influence on the Natural Desiccated Thyroid Market center primarily on optimizing individual patient dosing, predicting patient response to NDT versus synthetic options, and improving the consistency and tracking of the raw material supply chain. Users are keenly interested in how Artificial Intelligence and Machine Learning (ML) algorithms could address the long-standing challenge of NDT—variability. Specifically, patients and providers question if AI can analyze patient-specific genetic profiles, symptom severity data, and blood panel results to determine the optimal starting dose and titration schedule, moving beyond traditional trial-and-error methods. Furthermore, there is significant interest in applying AI to pharmaceutical quality control, specifically using computer vision and predictive analytics to verify the consistency of hormone content in porcine raw materials and finished drug products, thereby mitigating the risk of recalls and improving therapeutic reliability for patients who depend on stable NDT dosing. The general expectation is that AI will introduce precision medicine principles into a historically difficult-to-standardize therapeutic area, enhancing both safety and efficacy.
- AI-driven personalized dosing recommendations for NDT based on genetic markers and metabolic data.
- Enhanced quality control using machine learning algorithms to predict and detect batch-to-batch potency inconsistencies in manufacturing.
- Optimization of the raw material supply chain, ensuring consistent sourcing and minimizing environmental impacts through predictive logistics.
- Accelerated discovery and testing of combination therapies complementary to NDT for persistent hypothyroid symptoms.
- Chatbots and diagnostic support tools for patients seeking preliminary information on NDT eligibility and potential side effects.
- Improved pharmacovigilance by using AI to analyze real-world evidence and patient reported outcomes for long-term NDT safety monitoring.
DRO & Impact Forces Of Natural Desiccated Thyroid Market
The Natural Desiccated Thyroid Market operates under a dynamic interplay of factors where strong patient advocacy (Driver) clashes with inherent product standardization challenges (Restraint), creating specific growth avenues (Opportunity). The primary market driver is the clinical observation that a significant subset of hypothyroid patients, potentially due to polymorphisms in T3 metabolizing enzymes, does not achieve complete symptom resolution when treated solely with levothyroxine (T4), leading them to seek NDT for its crucial T3 component and associated co-factors. This patient-driven demand, coupled with strong support from integrative and functional medical communities, maintains the market's stability and growth trajectory. However, the most significant restraint is the regulatory skepticism stemming from the animal source, which leads to concerns over pathogen risk and, critically, the difficulty in guaranteeing exact hormone ratios across batches, resulting in periodic product recalls that damage patient and physician confidence. These factors are further complicated by external impact forces such as evolving pharmaceutical standards for purity and the competitive pricing pressure exerted by readily available, inexpensive synthetic alternatives.
Impact forces currently shaping the NDT market are multifactorial. Regulatory scrutiny acts as a significant external force, requiring manufacturers to invest heavily in advanced analytical methods (like HPLC and mass spectrometry) to demonstrate compliance, thereby increasing production costs. Furthermore, the growing trend toward personalized medicine presents an internal impact force; as diagnostics become more sophisticated, physicians are increasingly able to identify specific patient groups who benefit disproportionately from NDT, solidifying its niche status rather than pursuing mass-market appeal. The persistent patient-reported efficacy, despite the standardization hurdles, drives a positive feedback loop, ensuring continued demand. The opportunity landscape is defined by geographical expansion into less saturated markets, particularly in regions where integrated medicine is gaining traction, and the development of specialized delivery systems or purified versions of NDT that can mitigate the inconsistency risk, offering manufacturers a premium pricing structure and greater regulatory compliance.
Segmentation Analysis
The Natural Desiccated Thyroid Market is systematically segmented based on formulation type, the specific application or medical condition being treated, and the distribution channel through which the product reaches the end consumer. Analyzing these segments provides a clear understanding of market dynamics, patient preferences, and the commercial viability of different product types. Formulation segmentation is critical as it differentiates standard immediate-release tablets from specialized compounded formulations, which cater to patients requiring specific dose adjustments or allergenic-free fillers. Application segmentation reveals the market's core purpose, overwhelmingly focused on the long-term management of overt hypothyroidism. The distribution channel breakdown highlights the importance of traditional retail pharmacies alongside compounding pharmacies, which play a crucial role in providing tailored treatment options that standard commercially available products cannot meet.
The pharmaceutical consistency and therapeutic application of NDT necessitate careful analysis across these segments. The dominance of tablets reflects the established prescription practices and the convenience of mass production, while the growth in compounding demonstrates a market response to the need for personalized care, particularly for patients with unique absorption or sensitivity issues. Understanding the flow through distribution channels is essential for manufacturers, as compounding pharmacies often require specialized supply chains and regulatory handling. As medical practices trend towards holistic and personalized care, segments related to customized dosages and specialized application (e.g., severe refractory hypothyroidism) are expected to witness higher growth rates, driving innovation in manufacturing processes and quality control to ensure reproducible therapeutic outcomes across all patient groups.
- By Formulation
- Tablets/Capsules (Standardized Commercial Products)
- Compounded Preparations (Customized Dosages)
- By Application
- Hypothyroidism (Primary Treatment)
- Myxedema Coma (Emergency Use, less common)
- Nodular Thyroid Disease (Adjunctive Therapy)
- By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Compounding Pharmacies
- By Source
- Porcine Derived
- Bovine Derived (Minimal Market Share)
Value Chain Analysis For Natural Desiccated Thyroid Market
The value chain for the Natural Desiccated Thyroid Market begins with a critical upstream segment focused on the sourcing and initial processing of raw animal materials. This involves specialized animal husbandry (porcine farms) and slaughterhouses that must adhere to stringent hygienic standards, followed by the collection and drying of thyroid glands. The primary challenge upstream is the inherent variability in the hormone content of the glands, requiring sophisticated chemical assays (HPLC-UV or Mass Spectrometry) immediately upon collection to grade the raw material. Midstream activities involve the pharmaceutical manufacturing process, including extraction, purification, mixing with excipients, and compression into final dosage forms (tablets/capsules). This stage is highly regulated and requires rigorous quality control checkpoints, particularly focusing on standardization to meet pharmacopeial specifications for T4 and T3 content, which significantly influences the final product cost and stability. Due to the historical inconsistency issues, investment in analytical technology forms a major cost component in the midstream.
Downstream activities are dominated by distribution and patient access. The distribution channel is bifurcated: standard commercial NDT products move through wholesale distributors to retail and hospital pharmacies, following typical pharmaceutical logistics. Compounded NDT preparations, however, rely on a specialized distribution network involving dedicated compounding pharmacies that receive the raw pharmaceutical-grade thyroid extract (API) and prepare tailored prescriptions. The marketing and sales approach is unique, often targeting endocrinologists, general practitioners, and, crucially, functional and integrative medicine clinics. Direct-to-consumer communication, often through patient advocacy platforms and online resources, plays a significant role in driving prescription requests. The overall value chain is highly sensitive to regulatory fluctuations, raw material prices, and sustained patient preference, with high value added at the standardization and final compounding/dispensing stages.
Natural Desiccated Thyroid Market Potential Customers
The primary customer base for the Natural Desiccated Thyroid Market consists of individuals diagnosed with hypothyroidism, specifically those who exhibit persistent symptoms or a lack of clinical satisfaction despite achieving normal TSH levels while on standard levothyroxine (T4) therapy. These patients often possess specific metabolic profiles, such as polymorphisms in the deiodinase enzymes (DIO1, DIO2) responsible for converting T4 to the active T3 hormone, making direct T3 supplementation, as provided in NDT, therapeutically beneficial. This core group represents patients seeking a more holistic or biologically complete replacement hormone profile. Secondary, yet highly important, customers include endocrinologists and general practitioners who maintain an open approach to non-synthetic options, particularly those associated with functional or integrated medicine practices, where NDT is often the preferred initial treatment for symptomatic hypothyroidism, rather than a secondary resort. The high levels of patient engagement and self-education within this community mean the customers are often proactive in requesting specific brand or formulation types, dictating the therapeutic choices.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 450 Million |
| Market Forecast in 2033 | USD 610 Million |
| Growth Rate | 4.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Acella Pharmaceuticals, LLC (NP Thyroid), Allergan PLC (formerly Forest Laboratories/Actavis - Armour Thyroid), RLC Labs (Nature-Throid, Westhroid), Pfizer (Synthroid), Mylan N.V. (Generic Levothyroxine Players), Fresenius Kabi, Lannett Company, Sigma-Aldrich (Raw API Supplier), Thermo Fisher Scientific (Analytical Testing), Advanz Pharma, ANI Pharmaceuticals, Amneal Pharmaceuticals, Merck KGaA, AbbVie, West-Ward Pharmaceuticals. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Natural Desiccated Thyroid Market Key Technology Landscape
The manufacturing and quality control of Natural Desiccated Thyroid products are heavily reliant on advanced analytical and standardization technologies due to the inherent biological variability of the porcine source material. The key technological focus centers on ensuring precise quantification of active pharmaceutical ingredients (T4 and T3) in both the raw extract and the final dosage form. High-Performance Liquid Chromatography (HPLC) coupled with ultraviolet (UV) detection or, increasingly, Mass Spectrometry (MS) is the gold standard for separating and quantifying the exact concentration of active hormones, mitigating the risk of under- or over-dosing. Manufacturers are investing in high-throughput automated testing systems to conduct rigorous batch testing quickly and efficiently, moving beyond traditional, less precise methods. These analytical advancements are crucial for meeting modern regulatory requirements, particularly those enforced by the FDA, which have historically scrutinized NDT products for consistency.
Beyond analytical chemistry, process technology in the midstream production phase is critical. Specialized micro-encapsulation and granulating techniques are sometimes employed to improve the stability and consistency of the mixture before tableting, ensuring uniform distribution of the potent thyroid hormones throughout the pill matrix. Furthermore, manufacturers are exploring advanced sourcing technologies, including controlled animal farming and rapid preservation techniques for the porcine glands, aiming to minimize post-mortem degradation of the hormones before processing. Finally, data management systems utilizing predictive quality models are being integrated into manufacturing lines. These systems use historical data from raw material testing and finished product assays to predict potential batch failures, allowing for proactive adjustments in the mixing and compression stages, thereby significantly reducing waste and improving the reliability of the global NDT supply chain.
Regional Highlights
The global Natural Desiccated Thyroid market exhibits significant regional variations in terms of adoption, prescription rates, and regulatory acceptance, primarily due to differing healthcare philosophies and regulatory environments concerning non-synthetic, animal-derived hormones.
- North America (United States and Canada): This region dominates the NDT market, accounting for the largest share in terms of revenue and consumption. The dominance is attributed to a combination of high prevalence of hypothyroidism, strong patient advocacy groups (which actively promote NDT as a superior option for certain patients), and a robust functional and integrative medicine sector that heavily utilizes NDT. The U.S. market, specifically, hosts the major manufacturers and has a long history of NDT use, making brand recognition and physician familiarity high.
- Europe: The European market shows moderate, but cautious, growth. Regulations from the European Medicines Agency (EMA) and national health authorities tend to be stricter concerning animal-derived hormonal preparations, often favoring pure synthetic alternatives like levothyroxine (T4) and liothyronine (T3). NDT use is frequently confined to specialized clinics or is available via named-patient importation schemes, restricting widespread commercial access. However, countries like the UK and Germany are seeing increased interest from patients seeking alternative treatments.
- Asia Pacific (APAC): The APAC region represents the fastest-growing market, albeit from a smaller base. Growth is fueled by increasing healthcare expenditure, rising awareness of thyroid disorders, and the expansion of Western-style medical practices. Emerging economies like China and India are seeing a rise in lifestyle-related chronic diseases, including hypothyroidism. While traditional medicine remains strong, the adoption of specialized Western pharmaceuticals, including NDT for refractory cases, is gaining traction, particularly in private healthcare settings in Australia and South Korea.
- Latin America (LATAM): This region maintains a fragmented market structure. Adoption of NDT is often correlated with the presence of private healthcare systems and the influence of U.S. medical training on local practitioners. Brazil and Mexico show relatively higher demand for NDT compared to smaller nations, often relying on imported products or local compounding pharmacies. Regulatory pathways are diverse and complex, creating hurdles for consistent market penetration.
- Middle East and Africa (MEA): The MEA market is the smallest contributor but is expected to grow steadily. Growth drivers include improving diagnostic capabilities for endocrine disorders and increasing health investment, particularly in the Gulf Cooperation Council (GCC) countries. Cultural and religious considerations regarding porcine products can influence market acceptance in certain areas, requiring manufacturers to address consumer sensitivities proactively.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Natural Desiccated Thyroid Market, encompassing manufacturers of commercial products, key compounding suppliers, and dominant synthetic competitors influencing the overall market landscape.- Acella Pharmaceuticals, LLC (NP Thyroid)
- Allergan PLC (Part of AbbVie - Manufacturer of Armour Thyroid)
- RLC Labs, Inc. (Nature-Throid, Westhroid - currently experiencing supply issues or reformulation)
- Pfizer Inc. (Synthroid manufacturer, key competitor in synthetic T4)
- Merck KGaA (European thyroid medication provider)
- Lannett Company, Inc.
- ANI Pharmaceuticals, Inc.
- Mylan N.V. (A Viatris Company, significant generic manufacturer)
- Fagron N.V. (Key compounding supplier)
- PCCA (Professional Compounding Centers of America)
- Spectrum Chemical Mfg. Corp. (API and excipient supplier)
- West-Ward Pharmaceuticals (Generic T4)
- Perrigo Company plc
- Advanz Pharma Corp.
- AbbVie Inc. (Through acquisition of Allergan)
- Sigma-Aldrich (Raw material supplier for compounding)
- Thermo Fisher Scientific (Analytical testing services and equipment)
- Bausch Health Companies Inc.
- Teva Pharmaceutical Industries Ltd.
- Amneal Pharmaceuticals, Inc.
Frequently Asked Questions
Analyze common user questions about the Natural Desiccated Thyroid market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is Natural Desiccated Thyroid (NDT) and how does it differ from Levothyroxine?
NDT is a medication derived from porcine thyroid glands, containing both T4 (levothyroxine) and T3 (liothyronine) hormones, along with various thyroid co-factors. Levothyroxine (e.g., Synthroid) is a synthetic drug containing only the T4 hormone. NDT is often preferred by patients whose symptoms persist on T4-only therapy due to its comprehensive hormone profile, offering a more biologically complete replacement.
What are the primary regulatory challenges facing the NDT market?
The main challenge is maintaining stringent batch-to-batch consistency. Because NDT is animal-derived, hormone potency (specifically the ratio and concentration of T4 and T3) can naturally vary. Regulatory bodies, such as the FDA, demand rigorous quality control standards to ensure predictable dosing, leading to increased scrutiny and occasional product recalls when standardization requirements are not consistently met by manufacturers.
Which patient groups are most likely to benefit from Natural Desiccated Thyroid treatment?
Patients most likely to benefit are those diagnosed with primary hypothyroidism who continue to experience debilitating symptoms such as fatigue, depression, or weight fluctuations despite having laboratory TSH levels within the normal range while taking levothyroxine. This subset often includes individuals with impaired peripheral T4-to-T3 conversion capability, making direct T3 inclusion via NDT therapeutically advantageous for symptom resolution.
How is the global supply chain for NDT maintained given its porcine source?
The NDT supply chain relies on specialized sourcing networks that acquire dried thyroid glands from carefully selected porcine husbandry facilities, primarily in regulated environments. Manufacturers implement strict upstream quality control measures to grade the raw material based on hormone content before pharmaceutical processing. Geopolitical factors, animal health epidemics, and regulatory import/export restrictions all influence the stability and consistency of the international NDT supply chain.
What role do compounding pharmacies play in the Natural Desiccated Thyroid market?
Compounding pharmacies are essential providers of NDT, offering customized formulations. They address patient needs for specific, non-standard dosages, or provide preparations free of certain excipients (like dyes or gluten) found in commercial products, catering to patients with sensitivities or allergies. This channel meets the demand for truly personalized thyroid hormone replacement therapy when commercial options are unsuitable.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
