Neuromuscular Blocking Drug Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 434805 | Date : Dec, 2025 | Pages : 241 | Region : Global | Publisher : MRU
Neuromuscular Blocking Drug Market Size
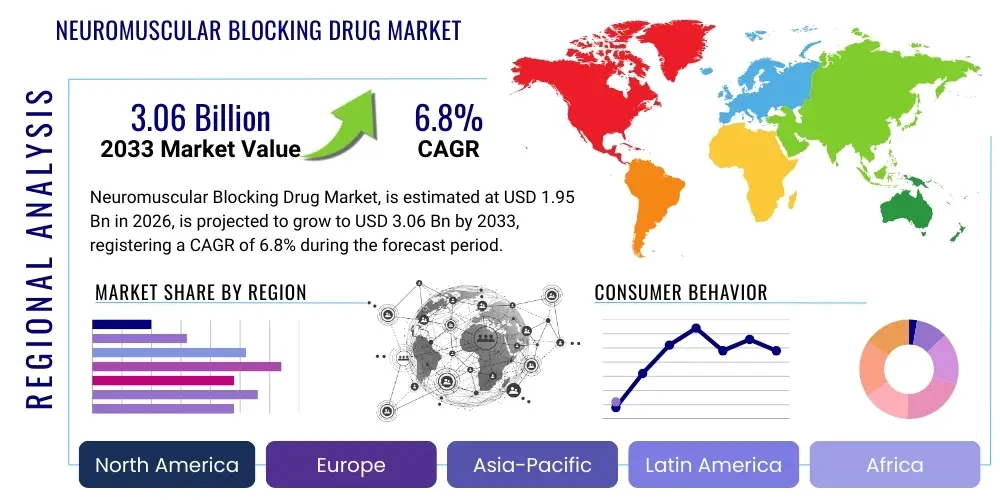
The Neuromuscular Blocking Drug Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2026 and 2033. The market is estimated at USD 1.95 Billion in 2026 and is projected to reach USD 3.06 Billion by the end of the forecast period in 2033. This consistent expansion is driven primarily by the escalating volume of surgical procedures performed globally, particularly complex surgeries requiring optimal muscle relaxation for procedural success, alongside advancements in anesthesia monitoring technologies that ensure safer administration and reversal of these critical agents.
The valuation reflects the critical role Neuromuscular Blocking Agents (NMBAs) play in modern operative medicine, spanning general surgery, critical care, and emergency medicine. While genericization of established drugs presents pricing pressures, the overall market growth is sustained by the introduction of innovative, fast-acting NMBAs and their corresponding reversal agents, such as sugammadex, which significantly enhance procedural throughput and patient recovery profiles. Furthermore, the increasing prevalence of chronic diseases necessitating surgical intervention, especially within aging populations in developed and rapidly developing economies, ensures a steady demand trajectory throughout the forecast period.
Neuromuscular Blocking Drug Market introduction
The Neuromuscular Blocking Drug Market encompasses pharmaceuticals specifically designed to induce temporary skeletal muscle paralysis, primarily utilized during general anesthesia to facilitate tracheal intubation and optimize surgical conditions. These essential medications interfere with the transmission of impulses between nerves and muscles at the neuromuscular junction, offering anesthesiologists precise control over patient mobility during invasive procedures. The product portfolio includes depolarizing agents, such as succinylcholine, and non-depolarizing agents, which are further classified based on their duration of action (short, intermediate, or long-acting), including prominent drugs like rocuronium, vecuronium, cisatracurium, and pancuronium. The clinical necessity for profound muscle relaxation in procedures ranging from cardiothoracic surgery to neurosurgery solidifies the market’s foundational importance in the healthcare landscape.
Major applications of NMBAs extend beyond the operating room into critical care settings, particularly for patients requiring mechanical ventilation to improve lung compliance and prevent patient-ventilator dyssynchrony. The primary benefits of these drugs are enhanced surgical field visibility, reduced risk of patient movement during delicate procedures, and facilitated rapid sequence intubation, thereby minimizing aspiration risk. Driving factors propelling this market include the global increase in both elective and emergency surgeries due to rising health awareness and access to surgical care, coupled with continuous innovation focused on minimizing side effects and optimizing recovery times. The development of selective relaxant binding agents (SRBAs), notably sugammadex, has revolutionized the field by offering rapid and complete reversal of certain NMBAs, dramatically improving patient safety profiles and reducing post-operative complications related to residual neuromuscular block (RNMB).
The market environment is characterized by stringent regulatory oversight, given the inherent risks associated with paralyzing agents, necessitating high standards for manufacturing and clinical usage. Technological advancements in neuromuscular monitoring (e.g., train-of-four monitoring) have become critical adjuncts, promoting safer and more accurate dosing, which, in turn, supports the expanded use of potent agents. Furthermore, the integration of these drugs into standardized Enhanced Recovery After Surgery (ERAS) protocols drives preference for agents offering predictable pharmacokinetics and rapid, reliable reversal. The ongoing competitive dynamics focus heavily on patent expiration strategies, geographic expansion, and securing preferential formulary status within major hospital systems, ensuring the continuous evolution of anesthetic practice and drug utilization patterns globally.
Neuromuscular Blocking Drug Market Executive Summary
The Neuromuscular Blocking Drug Market is defined by robust business trends centered on managing the trade-off between cost-effectiveness (driven by generics) and superior patient outcomes (driven by novel reversal agents). Key business trends indicate a significant market shift towards intermediate-acting, non-depolarizing agents, particularly those reversible by Sugammadex, due to improved patient safety and faster operating room turnaround times. Pharmaceutical companies are strategically focusing on lifecycle management, emphasizing the clinical superiority and economic benefits of combination therapies (NMBA + specific reversal agent) over traditional alternatives. Furthermore, there is a growing consolidation trend among specialized anesthesia drug manufacturers aimed at achieving economies of scale and enhancing penetration into emerging markets, where surgical volume growth is accelerating rapidly.
Regional trends reveal that North America and Europe currently dominate the market, largely attributed to sophisticated healthcare infrastructure, high per capita healthcare spending, and established guidelines mandating rigorous neuromuscular monitoring and effective reversal. However, the Asia Pacific (APAC) region is forecasted to exhibit the highest CAGR, primarily fueled by massive infrastructure investment in healthcare, increasing insurance penetration, and the rising burden of non-communicable diseases necessitating complex surgery in populous nations like China and India. Latin America and the Middle East & Africa (MEA) are emerging as critical growth areas, driven by expanding medical tourism and governmental initiatives aimed at improving access to advanced surgical care, though these regions still face challenges related to product affordability and supply chain stability.
Segment trends highlight the dominance of the Non-Depolarizing Agents segment, specifically rocuronium and its associated reversal agents, due to their favorable side effect profile and clinical versatility compared to depolarizing agents like succinylcholine, whose usage is increasingly restricted due to concerns regarding malignant hyperthermia risk. Among applications, general surgery maintains the largest market share, but the segment of Critical Care & Emergency Medicine is projected to witness the fastest growth, propelled by the increasing severity of respiratory failure cases globally, requiring prolonged mechanical ventilation and pharmacological paralysis. The segment of reversal agents is crucial, as the introduction of Sugammadex has fundamentally altered prescribing habits, making the pharmacoeconomic argument for its use compelling despite its higher cost, due to demonstrated improvements in patient outcomes and reduction in ICU stay duration.
AI Impact Analysis on Neuromuscular Blocking Drug Market
User inquiries regarding the impact of Artificial Intelligence (AI) on the Neuromuscular Blocking Drug Market overwhelmingly focus on two core themes: optimizing dosage precision and predicting adverse events, particularly residual paralysis. Common questions revolve around whether AI-powered anesthesia delivery systems can eliminate human error in NMBA administration, how machine learning algorithms can integrate real-time patient physiological data (e.g., EMG, TOF ratios) to determine optimal titration schedules, and the potential for AI models to predict patients susceptible to prolonged blockade or allergic reactions. Users express high expectations that AI will standardize best practices, reduce drug waste, and fundamentally transform safety protocols, while simultaneously raising concerns about the regulatory hurdles and clinical validation required for such critical, life-sustaining drug management systems.
AI is set to significantly enhance the precision and safety of neuromuscular blockade management by integrating complex data streams—including patient demographics, comorbidities, pharmacogenomics, and continuous physiological monitoring—into predictive dosing models. These advanced algorithms can dynamically adjust NMBA infusion rates to maintain the target depth of block, preventing both inadequate paralysis (risking patient movement) and excessive dosing (risking prolonged recovery). This level of personalized medicine minimizes the reliance on static guidelines, ensuring that the patient receives the minimum effective dose required for the surgical procedure. Such predictive analytics will drastically reduce the incidence of residual neuromuscular block (RNMB), a major concern associated with increased morbidity and mortality.
Furthermore, AI applications are extending into drug development and clinical trial optimization for next-generation NMBAs and reversal agents. Machine learning can rapidly analyze large datasets from preclinical studies to identify lead compounds with desirable pharmacokinetic properties, accelerating the path to market. In the clinical setting, AI tools are invaluable for continuous quality improvement by analyzing aggregated anesthetic records to identify patterns of suboptimal NMBA use or reversal failure across institutions. This diagnostic capability allows hospitals to refine protocols, train staff more effectively, and ensure strict adherence to safety mandates, thereby embedding AI as a critical layer of oversight and quality assurance within the anesthesia practice.
- AI-driven optimization of NMBA dosing via predictive pharmacokinetics and pharmacodynamics modeling.
- Real-time monitoring systems enhanced by machine learning to detect and prevent residual neuromuscular blockade (RNMB).
- Development of decision support systems for anesthesiologists to recommend optimal timing and dosage of reversal agents (e.g., Sugammadex).
- AI analysis of patient history and genetic markers to predict susceptibility to adverse drug reactions, such as anaphylaxis or prolonged block.
- Automation of anesthetic record-keeping and auditing for regulatory compliance and quality assurance improvement.
DRO & Impact Forces Of Neuromuscular Blocking Drug Market
The Neuromuscular Blocking Drug market is significantly influenced by a dynamic interplay of Drivers (D), Restraints (R), and Opportunities (O), creating distinct Impact Forces. Key drivers include the exponential increase in the global surgical volume, driven by aging populations and greater access to healthcare, alongside mandatory institutional guidelines emphasizing the use of reversal agents like Sugammadex to ensure patient safety and faster recovery times. Simultaneously, the market faces restraints such as significant pricing pressure due to the genericization of established NMBAs (e.g., rocuronium and vecuronium), the inherent risks of residual paralysis if monitoring is inadequate, and stringent regulatory requirements that delay the introduction of novel chemical entities. Opportunities arise from developing ultra-short-acting NMBAs suitable for outpatient procedures and integrating advanced monitoring technology (e.g., quantitative TOF monitoring) to improve clinical accuracy and drug utilization. The combined impact forces center on achieving a superior safety profile and improving operating room efficiency (throughput) as key differentiators in competitive strategy.
Detailed analysis of the Drivers reveals that technological integration, specifically the widespread adoption of quantitative neuromuscular monitoring devices (e.g., acceleromyography or electromyography), acts as a substantial growth catalyst. These devices validate the necessity of NMBAs and ensure their appropriate reversal, mitigating legal and clinical liabilities associated with RNMB. Furthermore, the rising incidence of trauma, cardiac ailments, and orthopedic conditions globally necessitates frequent surgical interventions, directly correlating with increased demand for both paralyzing agents and their antagonists. This demand is further amplified by the expansion of minimally invasive and robotic surgeries, which often require profound and stable muscle relaxation for optimal visualization and instrument manipulation. Strategic investment in training anesthesiologists in standardized monitoring practices also drives product uptake and responsible usage.
Conversely, major restraints relate to the financial sustainability of the market, particularly the high cost of patented reversal agents like Sugammadex, which often faces pushback from hospital procurement bodies and formulary committees, especially in budget-constrained systems. Furthermore, while extremely rare, the potential for life-threatening complications such as anaphylaxis associated with NMBAs necessitates cautious prescribing and contributes to clinical apprehension in certain patient demographics. The primary opportunity lies in pharmacogenomic research to personalize NMBA therapy, identifying individuals who metabolize these drugs differently, thus allowing for truly individualized medicine. Another significant opportunity involves expanding the utility of NMBAs beyond the operating theatre, particularly in the management of severe status epilepticus or in managing patients with acute respiratory distress syndrome (ARDS) requiring deep sedation and muscle relaxation for optimal lung protective ventilation strategies, solidifying the market's trajectory towards specialized critical care applications.
Segmentation Analysis
The Neuromuscular Blocking Drug market segmentation offers a granular view of product utilization and market dynamics, primarily categorized by Drug Type, Type of Blockade, Application, and Distribution Channel. This structural breakdown helps stakeholders understand where clinical demand is concentrated and how market penetration strategies need to be tailored. The fundamental split between depolarizing and non-depolarizing agents highlights differing safety profiles and application niches; for instance, depolarizing agents (like succinylcholine) maintain niche utility for rapid sequence induction, while non-depolarizing agents dominate general surgical practice due to their predictable pharmacokinetics and availability of effective reversal agents. The analysis of market share by duration of action—short, intermediate, and long-acting—is essential for optimizing surgical scheduling and operating room efficiency, with intermediate-acting drugs typically capturing the largest volume share.
Segmentation by Application reveals that the General Surgery segment, encompassing abdominal, orthopedic, and plastic procedures, holds the dominant market position due to the sheer volume of cases requiring endotracheal intubation and muscle relaxation. However, the Critical Care & Emergency Medicine segment is rapidly expanding, driven by the increasing need for mechanical ventilation in treating acute respiratory failure and severe sepsis, requiring prolonged NMBA infusions. Furthermore, the segmentation encompassing Reversal Agents, especially selective agents, is the fastest-growing component, reflecting a strong shift in clinical practice toward ensuring complete recovery from neuromuscular blockade before extubation, drastically reducing postoperative pulmonary complications and hospital stay duration. This growth underlines the premium placed on safety and outcome-focused drug selection by clinicians.
From a commercial perspective, segmentation by Distribution Channel—Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies—demonstrates the critical role of institutional purchasing, as NMBAs are overwhelmingly used in acute care settings and intensive care units, making hospital formularies the primary battleground for market penetration. Geographically, segmentation underscores the maturity differences between Western markets, characterized by high adoption rates of premium reversal agents and advanced monitoring, and emerging economies, where cost-effectiveness remains a decisive factor, often favoring generic, intermediate-acting NMBAs. Understanding these segmented dynamics allows market participants to develop targeted sales strategies, regulatory compliance plans, and R&D investment portfolios that align with specific clinical needs and regional economic realities.
- By Drug Type
- Depolarizing Agents (e.g., Succinylcholine)
- Non-Depolarizing Agents
- Benzylisoquinolinium Compounds (e.g., Cisatracurium, Atracurium)
- Steroidal Compounds (e.g., Rocuronium, Vecuronium, Pancuronium)
- By Duration of Action
- Short-Acting
- Intermediate-Acting (Dominant Segment)
- Long-Acting
- By Application
- General Surgery
- Cardiothoracic Surgery
- Neurosurgery
- Orthopedic Surgery
- Critical Care & Emergency Medicine
- By Reversal Agent
- Acetylcholinesterase Inhibitors (e.g., Neostigmine)
- Selective Relaxant Binding Agents (SRBAs) (e.g., Sugammadex)
Value Chain Analysis For Neuromuscular Blocking Drug Market
The Value Chain for the Neuromuscular Blocking Drug Market begins with rigorous Upstream Analysis, focusing on the sourcing and synthesis of complex Active Pharmaceutical Ingredients (APIs). Manufacturing NMBAs, particularly the highly sophisticated steroidal compounds like rocuronium, requires specialized chemical synthesis capabilities, stringent quality control to ensure purity and potency, and compliance with Good Manufacturing Practices (GMP). Key upstream stakeholders include specialized chemical suppliers and proprietary API manufacturers. Price fluctuations of raw materials and the complexity of synthesizing biologically active molecules are critical factors influencing upstream costs and supply stability. Companies maintaining integrated control over API production often possess a competitive advantage regarding cost containment and intellectual property protection, particularly for newer, patented molecules and their corresponding reversal agents, where synthesis complexity is significantly higher.
Midstream activities encompass formulation, sterile filling, packaging, and the highly regulated process of clinical trials and regulatory approval. Once the drugs are manufactured, the Downstream Analysis focuses on distribution channels, including both Direct and Indirect pathways. Direct distribution involves large pharmaceutical companies supplying NMBAs directly to centralized hospital procurement systems or governmental health services under long-term contracts. Indirect distribution relies heavily on specialized pharmaceutical wholesalers and distributors who manage cold chain logistics, inventory management, and regional last-mile delivery to smaller hospitals, ambulatory surgical centers, and clinics. Because NMBAs are injectable, temperature-sensitive drugs, maintaining the cold chain is paramount, adding complexity and cost to the logistics component of the value chain, making specialized distributors essential partners.
The final stage involves interaction with end-users, dominated by anesthesiologists and critical care physicians. The distribution channel is predominantly institutional; Hospital Pharmacies act as the primary intermediary between the distributor/manufacturer and the end-user. Sales success is heavily dependent on obtaining favorable formulary approval within major hospital networks, which often involves pharmacoeconomic studies demonstrating the cost-effectiveness (e.g., reduced ICU stay) of the drug, particularly for premium products like Sugammadex. Marketing and sales strategies are highly focused on clinical education, peer-to-peer influence, and demonstrating clinical superiority through outcomes data, rather than broad consumer marketing, solidifying the professional and specialized nature of the direct and indirect distribution networks within the highly concentrated institutional healthcare setting.
Neuromuscular Blocking Drug Market Potential Customers
The primary customers and end-users of Neuromuscular Blocking Drugs are institutional entities characterized by acute patient care environments, rather than individual consumers. This encompasses a broad spectrum of healthcare facilities where surgical procedures and intensive care management are performed. Specifically, major acute care hospitals, often categorized as tertiary or quaternary care centers, represent the largest volume purchasers, driven by their high surgical caseloads across specialties such as cardiovascular, neuro, orthopedic, and general surgery. These institutions prioritize drugs that offer the best balance of efficacy, safety (especially reliable reversal), and cost-effectiveness, making formulary inclusion a crucial factor for manufacturers. Furthermore, academic medical centers are key customers due to their involvement in clinical research, setting best practice standards, and extensive usage in complex, high-risk procedures.
Secondary potential customers include Ambulatory Surgical Centers (ASCs), which are increasingly important, especially in developed economies, due to the shift toward outpatient procedures. ASCs typically favor short-acting or intermediate-acting NMBAs with extremely rapid and reliable reversal profiles to ensure quick patient turnover and discharge, driving demand for products like rocuronium/sugammadex combinations. Additionally, specialized critical care units, including dedicated Intensive Care Units (ICUs) and Neonatal Intensive Care Units (NICUs), are significant customers. While the volume of NMBAs used in the ICU setting is lower than in the OR, the duration of use is often prolonged, necessitating different formulations and highly accurate infusion systems for continuous sedation and paralysis management in patients with acute respiratory failure or severe tetanus.
Beyond traditional healthcare facilities, governmental emergency medical services (EMS) and military hospitals also represent niche but important customer segments, particularly for rapid sequence induction agents required in trauma or field settings. Moreover, educational institutions and simulation centers purchase NMBAs for training purposes, allowing future anesthesiologists and nurses to practice safe drug administration and monitoring techniques. The purchasing decision across all these customer segments is highly influenced by regulatory guidelines, professional society recommendations (e.g., American Society of Anesthesiologists), clinical outcome data, and rigorous comparative economic analyses, positioning clinical evidence and safety data as paramount factors in customer acquisition and retention strategies.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 1.95 Billion |
| Market Forecast in 2033 | USD 3.06 Billion |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Merck & Co., Inc., AbbVie Inc., Sandoz (Novartis AG), GlaxoSmithKline plc, Mylan N.V., Teva Pharmaceutical Industries Ltd., Hikma Pharmaceuticals PLC, Fresenius SE & Co. KGaA, Viatris Inc., Pfizer Inc., Dr. Reddy's Laboratories Ltd., Aurobindo Pharma, Luitpold Pharmaceuticals Inc., Troikaa Pharmaceuticals Ltd., ICU Medical Inc., Sun Pharmaceutical Industries Ltd., Alvogen, Nexus Pharmaceuticals, Cumberland Pharmaceuticals Inc., and Zentiva Group a.s. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Neuromuscular Blocking Drug Market Key Technology Landscape
The technology landscape governing the Neuromuscular Blocking Drug market is primarily centered on precision, monitoring, and reversal efficacy, driving advancements in both pharmacological agents and their adjunct diagnostic tools. The most critical technological development impacting prescribing behavior is the introduction of Selective Relaxant Binding Agents (SRBAs), notably Sugammadex, which utilizes cyclodextrin chemistry to encapsulate steroidal NMBAs (like rocuronium or vecuronium), rapidly inactivating them in the plasma. This specific mechanism represents a pharmaceutical technology leap, offering a reliable, dose-dependent, and time-saving reversal that bypasses the limitations of traditional reversal agents (acetylcholinesterase inhibitors) which often cause significant cardiovascular side effects and are ineffective against profound blockade. This chemical innovation has redefined the standard of care for neuromuscular monitoring and reversal, fundamentally altering the perceived risk profile of certain NMBAs and facilitating safer anesthetic practice globally.
Equally vital is the technology used for monitoring the depth of neuromuscular blockade. Traditional qualitative monitoring (visual or tactile assessment) is being rapidly replaced by quantitative neuromuscular monitoring devices based on acceleromyography (AMG), kinemyography (KMG), or electromyography (EMG). These quantitative monitors provide objective, numerical measurements of the Train-of-Four (TOF) ratio, which is essential for guiding NMBA dosing and confirming complete recovery (TOF ratio > 0.9) before extubation. The widespread adoption of these monitors, often integrated into modern anesthesia machines, is driven by clinical guidelines emphasizing the correlation between accurate TOF measurements and reduced incidence of residual neuromuscular blockade (RNMB). The continuous refinement of these monitoring technologies—making them more portable, accurate, and easier to integrate into electronic health records—is a core technology trend sustaining market growth and promoting patient safety.
Further technological advancements include sophisticated drug delivery systems, such as Target-Controlled Infusion (TCI) pumps optimized for NMBAs, often incorporating algorithms that model patient pharmacokinetics to maintain a stable, targeted drug concentration. These systems enhance automated control over muscle relaxation, particularly crucial in prolonged procedures or in the ICU setting. Research and development efforts are also heavily invested in creating novel NMBAs that possess ultra-short durations of action, tailored specifically for brief, outpatient procedures, and exhibit minimized histamine release and cardiovascular effects. The technological focus remains on minimizing intraoperative instability, accelerating post-operative recovery, and leveraging smart technology to integrate drug administration decisions with real-time patient physiological responses, positioning the NMBA market at the intersection of pharmacology and advanced medical device integration.
Regional Highlights
Regional dynamics within the Neuromuscular Blocking Drug Market demonstrate substantial variation based on healthcare expenditure, surgical procedure volume, regulatory maturity, and the adoption rate of premium reversal agents. North America, encompassing the United States and Canada, currently holds the largest market share, predominantly due to its highly sophisticated healthcare infrastructure, high incidence of surgical procedures (both elective and non-elective), and the strong adherence to clinical guidelines recommending the use of quantitative monitoring and high-cost, selective reversal agents. The robust reimbursement mechanisms and the presence of major pharmaceutical innovators further solidify North America's dominance, characterized by high utilization rates of patented drugs such as Sugammadex, despite cost constraints.
Europe represents the second largest market, exhibiting mature utilization patterns similar to North America, especially within Western European nations (Germany, UK, France). Market growth in Europe is steady, driven by advancements in anesthetic protocols and increasing regulatory pressure to eliminate residual neuromuscular block. However, pricing and market access are often more tightly controlled by national health services (e.g., NHS in the UK), leading to a higher penetration of generic NMBAs, while reversal agent selection remains a critical area of market competition. Eastern Europe, conversely, offers emerging growth potential as healthcare infrastructure modernizes and access to standardized surgical care expands, progressively shifting away from older, long-acting NMBAs towards intermediate-acting alternatives.
The Asia Pacific (APAC) region is projected to experience the fastest growth during the forecast period. This accelerated growth is primarily attributed to rapidly improving healthcare accessibility, the vast and growing geriatric population requiring surgical interventions, and increasing governmental investments in modernizing hospital facilities in countries like China, India, and South Korea. While pricing sensitivity remains high, leading to a strong preference for cost-effective generic NMBAs, the awareness and adoption of safer anesthetic practices are increasing, signaling future growth in the segment of patented reversal agents. Latin America and the Middle East & Africa (MEA) represent nascent markets, driven by medical tourism and urbanization, but growth is often hampered by inconsistent regulatory environments and challenges related to the cold chain logistics required for temperature-sensitive injectable pharmaceuticals.
- North America: Market leader; characterized by high procedure volume, advanced monitoring adoption, and premium pricing for patented reversal agents (Sugammadex dominance).
- Europe: Mature market; strong focus on safety protocols and quality improvement; prevalence of generic utilization balanced by stringent guidelines on residual blockade management.
- Asia Pacific (APAC): Fastest growing region; exponential growth driven by infrastructure development, rising surgical caseloads, and improving access to standardized care, though pricing is a significant factor.
- Latin America & MEA: Emerging markets; growth driven by healthcare investment and medical tourism; challenged by complex logistics and price sensitivity.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Neuromuscular Blocking Drug Market.- Merck & Co., Inc.
- AbbVie Inc.
- Sandoz (Novartis AG)
- GlaxoSmithKline plc
- Mylan N.V.
- Teva Pharmaceutical Industries Ltd.
- Hikma Pharmaceuticals PLC
- Fresenius SE & Co. KGaA
- Viatris Inc.
- Pfizer Inc.
- Dr. Reddy's Laboratories Ltd.
- Aurobindo Pharma
- Luitpold Pharmaceuticals Inc.
- Troikaa Pharmaceuticals Ltd.
- ICU Medical Inc.
- Sun Pharmaceutical Industries Ltd.
- Alvogen
- Nexus Pharmaceuticals
- Cumberland Pharmaceuticals Inc.
- Zentiva Group a.s.
Frequently Asked Questions
Analyze common user questions about the Neuromuscular Blocking Drug market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary factor driving the growth of the Neuromuscular Blocking Drug market?
The principal driver is the globally increasing volume of surgical procedures requiring general anesthesia and muscle relaxation, coupled with the mandatory adoption of advanced reversal agents (like Sugammadex) and quantitative monitoring technologies to enhance patient safety and reduce the incidence of post-operative pulmonary complications associated with residual blockade.
How has the introduction of Sugammadex impacted the market for Neuromuscular Blocking Agents (NMBAs)?
Sugammadex, a selective relaxant binding agent, has significantly impacted the market by offering rapid and complete reversal of steroidal NMBAs (Rocuronium and Vecuronium). This shift has favored the use of these intermediate-acting NMBAs, leading to improved operating room efficiency and patient outcomes, despite the reversal agent's relatively high cost.
Which segment of the Neuromuscular Blocking Drug market is expected to exhibit the fastest growth?
The fastest growth is anticipated in the Critical Care & Emergency Medicine segment, driven by the increasing global prevalence of acute respiratory failure and severe critical illnesses requiring prolonged mechanical ventilation and pharmaceutical muscle relaxation. Additionally, the segment dedicated to Selective Reversal Agents is also growing rapidly due to heightened safety requirements.
What are the main restraints affecting the commercial growth of the NMBA market?
Major restraints include intense pricing pressure due to the widespread availability and use of generic versions of established NMBAs, the significant cost barriers associated with premium reversal agents like Sugammadex in budget-sensitive regions, and the continuous clinical challenge of mitigating the risk of residual neuromuscular block despite monitoring efforts.
What role does quantitative monitoring technology play in the Neuromuscular Blocking Drug market landscape?
Quantitative monitoring, such as acceleromyography (AMG), is crucial as it provides objective measurement of the depth of muscle paralysis (TOF ratio). This technology ensures precise dosing, minimizes the risk of overdose or residual blockade, and facilitates the appropriate timing and dosage of reversal agents, thereby serving as a foundational technology for safe NMBA administration.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager