Newcastle Disease Vaccine Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 433658 | Date : Dec, 2025 | Pages : 255 | Region : Global | Publisher : MRU
Newcastle Disease Vaccine Market Size
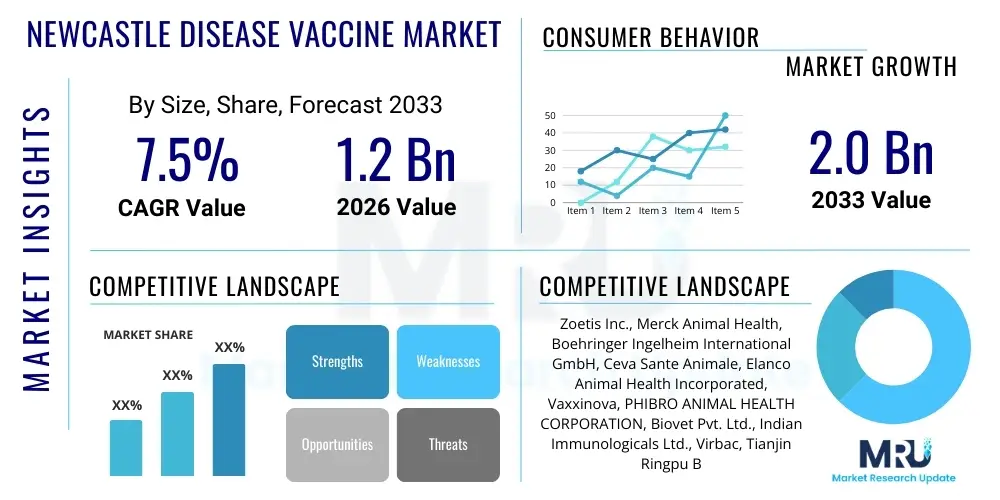
The Newcastle Disease Vaccine Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.5% between 2026 and 2033. The market is estimated at USD 1.2 Billion in 2026 and is projected to reach USD 2.0 Billion by the end of the forecast period in 2033. This substantial growth is primarily driven by the escalating global demand for poultry products, particularly in rapidly developing economies in the Asia Pacific and Latin America, coupled with mandatory government vaccination programs aimed at controlling widespread viral outbreaks. The economic imperative of protecting large-scale commercial poultry operations from highly contagious and economically devastating diseases like Newcastle Disease (ND) provides a robust foundation for continuous market expansion, necessitating investments in advanced vaccine technologies and efficient cold chain logistics globally. Furthermore, the persistent threat posed by velogenic and mesogenic strains of the Newcastle Disease Virus (NDV) mandates regular updates to vaccine compositions and administration strategies, thereby sustaining product lifecycles and encouraging innovation among key market players.
Newcastle Disease Vaccine Market introduction
The Newcastle Disease Vaccine Market encompasses the development, production, and distribution of biological preparations used to immunize poultry against the highly contagious avian viral disease, Newcastle Disease (ND). This disease, caused by the Newcastle Disease Virus (NDV), poses a catastrophic threat to the global poultry industry, leading to high mortality rates, severe economic losses, and restrictions on international trade. The primary products within this market include live attenuated vaccines, inactivated vaccines, and increasingly, modern recombinant vector vaccines. Live attenuated vaccines, such as LaSota and B1 strains, are widely favored for mass administration via drinking water or spray, offering rapid, broad immunity, while inactivated vaccines provide higher antibody titers and are often used for booster immunization in breeder flocks. Recombinant vaccines represent the cutting edge, engineered to express protective NDV antigens using safe viral or bacterial vectors, promising improved safety profiles and reduced cold chain dependency. Major applications involve routine prophylactic immunization in broiler chickens, layer hens, and breeding stock across commercial and small-scale farms globally, ensuring flock health and minimizing the risk of devastating outbreaks that can cripple food supply chains. The immediate benefit of these vaccines is the prevention of disease symptoms, reduction in viral shedding, and enhancement of overall productivity and food security. The driving factors fueling this market are predominantly the steep increase in global per capita consumption of chicken meat and eggs, stringent governmental regulations enforcing vaccination mandates, heightened biosecurity concerns among large poultry integrators, and continuous research into developing thermostable vaccines suitable for distribution in challenging tropical environments. Additionally, the rapid pace of urbanization and the subsequent shift towards industrialized poultry farming necessitate scalable and effective vaccination solutions to manage denser animal populations.
Newcastle Disease Vaccine Market Executive Summary
The Newcastle Disease Vaccine Market is characterized by robust business trends centered on technological advancements and geographic expansion. The market exhibits a clear shift towards recombinant and vector-based vaccines, which offer superior stability, reduced need for multiple dosing, and enhanced protection against emerging virulent strains of NDV. Key pharmaceutical companies are strategically focusing on acquiring regional manufacturing capabilities, particularly in high-growth Asia Pacific nations, to streamline supply chains and mitigate the complexities associated with global cold chain maintenance. Consolidation among smaller regional manufacturers and large multinational animal health companies is a prevailing trend, driving economies of scale in R&D and production. Furthermore, digital integration, leveraging data analytics and AI, is becoming crucial for optimizing vaccination schedules and predicting localized outbreak risks, transforming the operational efficiencies of veterinary practices and commercial farms. This emphasis on digitalization facilitates more precise and timely market responsiveness, particularly in densely populated poultry regions.
From a regional perspective, Asia Pacific maintains its dominance as the largest and fastest-growing market segment. This supremacy is directly attributable to the immense volume of poultry production, particularly in countries like China, India, and Indonesia, coupled with the high endemicity of Newcastle Disease and proactive government health initiatives aimed at stabilizing domestic food sources. North America and Europe, while representing mature markets, contribute significantly to market value through high investment in premium, technologically advanced recombinant vaccines and strict adherence to biosecurity protocols. Growth in these developed regions is slower but driven by innovation, focusing on vaccine quality and ease of administration. Conversely, Latin America and the Middle East & Africa are emerging as significant growth poles, fueled by rapid industrialization of poultry farming and increasing consumer demand for affordable protein sources, prompting governments to allocate substantial budgets towards comprehensive vaccination campaigns to protect nascent industries.
Segmentation trends highlight the continued dominance of the Live Attenuated segment by volume, favored for its low cost and ease of mass application via drinking water in commercial broiler operations. However, the Recombinant Vaccines segment is projected to exhibit the highest CAGR, reflecting the industry’s preference for efficacy, safety, and reduced biological reactivity. In terms of end-users, large commercial poultry farms remain the primary consumers, demanding specialized high-volume products and integrated health management solutions. The route of administration segmentation emphasizes the increasing adoption of drinking water and spray methods due to their non-invasive nature and suitability for treating large flocks efficiently. Product differentiation is increasingly reliant on developing multi-valent vaccines that simultaneously protect against ND and other prevalent avian diseases, simplifying farmer protocols and maximizing return on investment.
AI Impact Analysis on Newcastle Disease Vaccine Market
User queries regarding the impact of Artificial Intelligence (AI) on the Newcastle Disease Vaccine Market frequently revolve around optimizing diagnostic speed, improving vaccine development cycles, enhancing supply chain precision, and predicting disease outbreaks with greater accuracy. Common concerns center on how machine learning algorithms can rapidly analyze complex epidemiological data—such as viral genomic sequences and environmental factors—to identify potential mutations that might render current vaccines ineffective, thereby demanding quicker formulation updates. Users are keen to understand how AI-driven predictive modeling can transition from passive data collection to proactive, geographically tailored vaccination strategy recommendations, minimizing vaccine wastage and maximizing population immunity coverage. Furthermore, significant interest lies in AI's role in drug discovery, specifically in designing novel antigen candidates or delivery systems that can withstand varying environmental temperatures, a critical challenge in low-resource settings. The overarching expectation is that AI will dramatically reduce the time and cost associated with vaccine research, production optimization, and precise deployment, shifting the industry paradigm from reactive disease control to predictive health management, ultimately enhancing the return on investment for poultry producers and national food security agencies.
- AI-driven genomics analysis accelerates the identification and characterization of emerging virulent NDV strains, facilitating rapid vaccine updates.
- Machine learning algorithms optimize vaccine production yields and quality control by analyzing bioreactor parameters and manufacturing variables.
- Predictive modeling forecasts localized outbreak severity and timing, allowing veterinary services to deploy vaccines preemptively and efficiently.
- AI optimizes cold chain logistics and inventory management by mapping temperature fluctuations and demand patterns, minimizing spoilage and ensuring vaccine viability.
- AI assists in analyzing large clinical trial datasets to predict vaccine efficacy in diverse poultry populations and environmental conditions.
DRO & Impact Forces Of Newcastle Disease Vaccine Market
The dynamics of the Newcastle Disease Vaccine Market are shaped by a complex interplay of Drivers, Restraints, and Opportunities (DRO), which collectively constitute the principal Impact Forces influencing market direction and growth trajectories. A primary driver is the exponentially rising global demand for affordable protein sources, specifically chicken meat and eggs, which necessitates larger, more concentrated poultry farming operations. These dense environments inherently increase the risk of infectious disease transmission, mandating rigorous vaccination schedules and robust biosecurity protocols enforced by both commercial integrators and governmental agricultural bodies. Secondly, stringent regulatory frameworks and mandatory vaccination programs enacted by global bodies like the OIE (World Organisation for Animal Health) and national veterinary services ensure a sustained baseline demand for ND vaccines. Furthermore, continuous advancements in biotechnology, particularly the development of recombinant and thermostable vaccines, significantly broaden the applicability and efficacy of prophylactic treatments in varied geographical climates, overcoming traditional distribution challenges and attracting higher investment into R&D. These cumulative drivers exert a substantial upward force on the market, encouraging continuous product innovation and capacity expansion among leading market participants.
However, the market faces significant restraints that temper its growth potential. The most critical constraint is the necessity of maintaining a robust and uninterrupted cold chain infrastructure for transporting and storing traditional live and inactivated vaccines. Temperature excursions render vaccines inactive, leading to massive financial losses and ineffective disease control, a challenge particularly acute in remote or developing regions with unreliable electricity supply. Moreover, the complexities associated with vaccine administration, requiring skilled veterinary personnel and multiple dosing schedules throughout a bird's life cycle, increase operational costs and potential for administration errors. Public perception and skepticism regarding mass animal vaccination, although less pronounced than in human health, occasionally pose regulatory hurdles or slow down adoption rates in certain sophisticated consumer markets. Finally, the ability of NDV to rapidly mutate necessitates constant surveillance and frequent adjustments to vaccine strains, imposing high R&D costs and increasing the risk of vaccine failure if surveillance is inadequate or slow, potentially eroding farmer confidence in available products.
Opportunities for growth are concentrated primarily in the development and commercialization of next-generation vaccine technologies. This includes the push for 'needle-free' administration methods, such as in-ovo vaccination technology, which automates delivery and ensures precise dosing before hatch, offering maximum protection from day one and reducing handling stress. The creation of thermostable or heat-tolerant vaccines (often achieved through lyophilization or novel encapsulation techniques) promises to revolutionize distribution in regions where cold chain integrity is compromised, opening vast underserved rural markets. Additionally, the increasing trend towards multi-valent vaccines, which offer protection against ND alongside Infectious Bronchitis (IB) or Infectious Bursal Disease (IBD) in a single dose, simplifies logistics for farmers and enhances compliance. The convergence of veterinary medicine with digital health platforms, incorporating AI and IoT for real-time monitoring and dosage management, presents a substantial opportunity for companies positioned to offer integrated health management solutions rather than just standalone vaccine products. These opportunities allow market players to mitigate existing cold chain restraints and leverage technological superiority to achieve significant market share gains, particularly in emerging industrial poultry sectors.
Segmentation Analysis
The Newcastle Disease Vaccine Market is extensively segmented based on Type, Strain, Route of Administration, and End-User, reflecting the diverse requirements of the global poultry industry. The segmentation by Type is critical, differentiating between traditional approaches (Live Attenuated and Inactivated) and advanced biotechnical products (Recombinant Vaccines), each offering distinct advantages regarding immunity duration, safety profile, and cost-effectiveness. Strain segmentation (e.g., LaSota, B1, and various circulating field strains) is crucial for ensuring regional efficacy, as vaccines must be matched against endemic viral challenges. Route of Administration focuses on logistical efficiency, with mass administration methods (drinking water, spray) dominating high-volume commercial production, while parenteral (injection) routes are reserved for high-value breeder flocks. Finally, End-User classification separates large Commercial Farms, which demand high-volume, automated solutions, from small-scale Backyard Poultry operations, which often require thermostable, easily administered products. This granular segmentation allows manufacturers to tailor product development and marketing strategies to meet specific operational and epidemiological requirements globally.
- Type:
- Live Attenuated Vaccines (e.g., LaSota, B1)
- Inactivated Vaccines
- Recombinant Vaccines (e.g., Fowlpox Vector, Herpesvirus of Turkey (HVT) Vector)
- Strain:
- LaSota
- B1
- Mukteswar
- Other Strains (e.g., Komarov, Ulster)
- Route of Administration:
- Parenteral (Injection - Subcutaneous or Intramuscular)
- Ocular/Nasal Drops
- Drinking Water
- Spray/Aerosol
- In-Ovo (Increasingly utilized in automated hatcheries)
- End-User:
- Commercial Poultry Farms (Broilers, Layers, Breeders)
- Backyard and Small-Scale Poultry Keepers
- Government and Research Institutions
Value Chain Analysis For Newcastle Disease Vaccine Market
The value chain of the Newcastle Disease Vaccine Market initiates with upstream activities centered on extensive research and development (R&D) and the procurement of highly specialized raw biological materials. Upstream analysis involves sourcing specific, certified pathogen-free chicken embryos or cell lines required for viral propagation, alongside high-grade adjuvants, stabilizing agents, and specialized culture media. R&D investments are paramount, focusing on viral antigen selection, reverse genetics techniques for creating stable seed viruses, and process optimization to ensure high-titer vaccine output. Quality control at this stage, particularly pathogen screening and genetic stability testing, is strictly regulated and capital-intensive, forming a significant entry barrier. The successful synthesis and purification of the active pharmaceutical ingredient (API) in controlled bioprocessing facilities determines the foundational quality and yield of the final vaccine product, requiring adherence to Current Good Manufacturing Practices (cGMP) guidelines.
Midstream processes focus on large-scale manufacturing, formulation, and primary packaging. This phase includes the critical formulation step, where the purified antigen is combined with stabilizers and, for inactivated vaccines, adjuvants to enhance the immune response. Lyophilization (freeze-drying) processes are increasingly utilized, particularly for live attenuated vaccines, to enhance stability and longevity. Packaging involves specialized sterile glass or plastic vials, often requiring strict adherence to cold chain integrity, particularly during bulk storage prior to distribution. Distribution channels are highly specialized due to the thermal sensitivity of most ND vaccines. The channel structure involves a mix of Direct and Indirect approaches. Direct channels often involve large multinational pharmaceutical companies selling directly to major commercial poultry integrators or governmental procurement agencies, facilitating closer control over cold chain logistics and offering integrated veterinary technical support services. Indirect channels utilize specialized veterinary distributors, wholesalers, and licensed pharmacies, particularly for reaching smaller farms and backyard poultry keepers, relying on established regional cold chain transport networks.
Downstream analysis covers marketing, sales, veterinary administration, and end-user uptake. Sales strategies must be highly educational, requiring detailed technical support provided by veterinary specialists to farmers regarding proper handling, storage, timing, and administration techniques to ensure maximum efficacy. The final consumption stage involves the poultry producers themselves, who are responsible for integrating the vaccines into a comprehensive flock health management program. Efficacy tracking and post-marketing surveillance are essential components of the downstream value chain, providing crucial feedback loops to R&D and manufacturing teams regarding circulating strain match and product performance in field conditions. The successful execution of the downstream logistics, particularly maintaining the cold chain until the point of administration (the 'last mile'), is the most challenging and critical element determining the vaccine's actual effectiveness and preventing economic losses for the end-user.
Newcastle Disease Vaccine Market Potential Customers
The primary consumers and potential customers for Newcastle Disease Vaccines are entities deeply involved in poultry production, ranging from large-scale, vertically integrated agricultural corporations to individual smallholder farmers and governmental regulatory bodies. Commercial Poultry Farms represent the largest volume consumers. These operations, categorized into broiler production (meat), layer production (eggs), and breeding stock (parent and grandparent lines), necessitate high-volume, standardized vaccination protocols to protect massive, dense populations. Broiler operations typically utilize cost-effective, mass-administered live attenuated vaccines, while layers and breeders—representing higher economic value and longer productive lifecycles—often require premium inactivated or recombinant vaccines to ensure prolonged, robust immunity and protect subsequent generations. These commercial entities prioritize automation in delivery (e.g., in-ovo and drinking water systems), logistical reliability, and integrated veterinary consultation services.
A secondary, but rapidly expanding, customer base includes Smallholder and Backyard Poultry Keepers, particularly prevalent in Asia Pacific and Africa. While these customers purchase smaller volumes, their aggregate demand is significant, often requiring vaccines that are extremely thermostable, simple to administer (e.g., tablet or pellet formulations), and accessible through local veterinary clinics or governmental subsidized programs. Government Agencies, including Ministries of Agriculture and Animal Health Departments, constitute another critical customer segment. These bodies are major purchasers, responsible for national immunization programs, controlling endemic disease, and maintaining biosecurity for international trade compliance. Their purchasing decisions are often influenced by public health mandates, epidemiological surveillance data, and tenders focused on product cost-effectiveness, large-scale supply capacity, and compatibility with national cold chain infrastructure. Furthermore, organizations managing large poultry genetic conservation projects or research facilities also form a niche market for specialized, high-purity vaccines and diagnostic kits.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 1.2 Billion |
| Market Forecast in 2033 | USD 2.0 Billion |
| Growth Rate | 7.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Zoetis Inc., Merck Animal Health, Boehringer Ingelheim International GmbH, Ceva Sante Animale, Elanco Animal Health Incorporated, Vaxxinova, PHIBRO ANIMAL HEALTH CORPORATION, Biovet Pvt. Ltd., Indian Immunologicals Ltd., Virbac, Tianjin Ringpu Bio-Technology Co., Ltd., Qinhai Biotech, HVN (Hester Biosciences), Venky's (India) Ltd., FATRO S.p.A., Intervet International B.V. (Merck), Lohmann Animal Health, Agri Laboratories Ltd., Colorado Serum Company, Avian Biologicals. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Newcastle Disease Vaccine Market Key Technology Landscape
The technological landscape of the Newcastle Disease Vaccine Market is rapidly evolving, driven by the need for enhanced efficacy, reduced handling costs, and improved stability. Traditional vaccine production relies heavily on embryonated chicken eggs for viral propagation, a mature but time-intensive technology. However, the industry is increasingly adopting advanced genetic engineering techniques to combat viral mutation and enhance safety profiles. Reverse Genetics (RG) technology is now a cornerstone, allowing researchers to precisely manipulate the NDV genome to create highly immunogenic yet fully safe vaccine strains, eliminating virulence factors while preserving protective antigens. This precision facilitates the rapid production of updated strains tailored to specific regional outbreak challenges, providing a swift response capability previously unavailable.
Furthermore, the shift towards Recombinant Vector Vaccines represents the most significant technological leap. Vaccines utilizing non-pathogenic vectors, such as the Herpesvirus of Turkey (HVT) or Fowlpox virus, are engineered to express the critical F or HN proteins of NDV. HVT-vectored vaccines, in particular, offer superior, long-lasting immunity with a single administration (often in-ovo or at-hatch) and crucially, these DNA-based platforms are entirely unaffected by maternal antibodies present in young chicks, a common challenge for traditional live vaccines. This technology simplifies administration, reduces stress on the poultry, and offers excellent thermal stability compared to conventional live products, mitigating the stringent cold chain requirements that burden logistics, especially in tropical climates. The ability to combine multiple antigens into a single vector also paves the way for effective multi-valent vaccines, improving overall flock health management efficiency.
An auxiliary, yet vital, technological development is the implementation of advanced formulation science, particularly focusing on thermostabilization and novel delivery systems. Lyophilization techniques have been refined to extend the shelf life of live attenuated vaccines dramatically under non-refrigerated conditions. Additionally, new delivery methods like In-Ovo Vaccination systems use automated machinery to inject vaccines into the egg before hatching, ensuring every bird receives precise, early immunization, which is critical for maximizing productivity in large-scale hatcheries. Combined with Next-Generation Sequencing (NGS) technologies used for real-time epidemiological surveillance—monitoring NDV evolution and informing prompt vaccine strain selection—these technological advancements ensure that the market remains responsive, highly specialized, and capable of meeting the escalating biosecurity demands of the global poultry industry. The integration of high-throughput screening and analytical methods ensures that every batch manufactured meets the highest standards of purity and potency before deployment.
Regional Highlights
The global Newcastle Disease Vaccine market exhibits distinct regional dynamics, largely defined by the concentration of poultry production, regulatory environments, and the economic capacity for cold chain infrastructure investment. Asia Pacific (APAC) dominates the market, both in terms of consumption volume and future growth trajectory. This region, encompassing major producers like China, India, Indonesia, and Thailand, hosts the largest global poultry population. The endemic nature of highly virulent NDV strains necessitates continuous, often mandatory, vaccination programs. The rapid transition from backyard farming to large-scale commercial integration in these economies drives massive demand for scalable, cost-effective vaccines (primarily live attenuated), alongside a growing, though smaller, demand for premium recombinant options in sophisticated integrator operations. Government procurement plays a crucial role in APAC, focusing on mass immunization drives to protect local food security, making logistical efficiency and cold chain robustness paramount concerns for suppliers operating in the highly diverse climates of this region. The sheer size of the target animal population and the persistent presence of the disease underpin the leading role of APAC.
North America (NA) and Europe represent highly mature, value-driven markets. While poultry volumes are lower than in APAC, the market value is sustained by high adoption rates of advanced, often more expensive, recombinant vector vaccines (such as HVT-NDV). In these regions, the focus is less on basic disease control and more on maximizing productivity, improving animal welfare outcomes, and complying with stringent traceability and biosecurity standards. European countries adhere to strict regulations concerning animal health and feed safety, driving demand for vaccines with high safety profiles and robust efficacy claims. R&D innovation is concentrated here, with many major animal health companies utilizing these regions as testing grounds for next-generation administration technologies, such as automated in-ovo systems. The reliance on sophisticated veterinary services ensures high compliance rates, though the market growth is moderated by stable poultry populations and already established disease control protocols.
Latin America (LATAM) and the Middle East & Africa (MEA) are characterized as high-potential emerging markets. LATAM, particularly Brazil and Mexico, are major global exporters of poultry, resulting in substantial investment in biosecurity and high-quality vaccine procurement to maintain trade compliance and secure export markets. The transition from small-scale farming to industrial integration is accelerating, spurring significant demand for efficient, scalable vaccination solutions. In the MEA region, the challenge lies in overcoming infrastructural hurdles, particularly cold chain limitations in vast, often arid, geographies. Market growth here is heavily reliant on international aid, governmental subsidies, and the successful deployment of thermostable vaccine technologies to reach remote rural populations. Both regions demonstrate a strong CAGR prospect, driven by population growth, urbanization, and the corresponding industrialization of local food production systems, making them critical targets for companies looking to expand their global footprint beyond established markets.
- Asia Pacific: Largest market share by volume; driven by high poultry population, endemic disease, and government mandates; strong preference for cost-effective live vaccines.
- North America: Focus on premium recombinant vaccines and advanced in-ovo delivery systems; mature, value-driven market characterized by strict biosecurity and regulatory standards.
- Europe: Stable market, dominated by adherence to strict animal welfare standards; high adoption of advanced, safe vaccines; R&D hub for novel delivery platforms.
- Latin America: High growth due to significant poultry export volumes (especially Brazil); increasing industrialization mandates investment in quality vaccines for trade protection.
- Middle East & Africa: Fastest growing emerging market; primary challenge is cold chain infrastructure; high demand for thermostable and easy-to-administer vaccines to stabilize local food supply.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Newcastle Disease Vaccine Market.- Zoetis Inc.
- Merck Animal Health (Intervet International B.V.)
- Boehringer Ingelheim International GmbH
- Ceva Sante Animale
- Elanco Animal Health Incorporated
- Vaxxinova
- PHIBRO ANIMAL HEALTH CORPORATION
- Biovet Pvt. Ltd.
- Indian Immunologicals Ltd.
- Virbac
- Tianjin Ringpu Bio-Technology Co., Ltd.
- Qinhai Biotech
- HVN (Hester Biosciences)
- Venky's (India) Ltd.
- FATRO S.p.A.
- Lohmann Animal Health
- Agri Laboratories Ltd.
- Colorado Serum Company
- Avian Biologicals
- Biogenesis Bago S.A.
Frequently Asked Questions
Analyze common user questions about the Newcastle Disease Vaccine market and generate a concise list of summarized FAQs reflecting key topics and concerns.What are the primary types of Newcastle Disease Vaccines available globally?
The global market primarily utilizes three types of vaccines: Live Attenuated Vaccines (offering broad, rapid immunity suitable for mass administration); Inactivated Vaccines (providing high, long-lasting antibody titers, often used in breeder stock); and Recombinant Vaccines (advanced formulations offering superior safety, stability, and protection against maternal antibodies, typically administered in-ovo or at-hatch).
Why is the cold chain management a critical restraint in the Newcastle Disease Vaccine market?
Cold chain management is critical because most traditional live and inactivated ND vaccines are thermolabile (sensitive to heat). Failure to maintain consistent refrigeration (typically 2-8°C) during storage and distribution renders the vaccine inactive, leading to ineffective immunization, economic loss, and potential disease outbreaks. This is a major logistical challenge, particularly in developing regions with unreliable electricity or infrastructure.
Which geographical region dominates the Newcastle Disease Vaccine Market, and what drives this dominance?
The Asia Pacific (APAC) region dominates the market. This dominance is driven by the sheer volume of poultry production (China, India, Indonesia), the high endemicity of virulent NDV strains, and the mandatory, large-scale government vaccination programs required to secure national food supplies and control widespread outbreaks.
How are recombinant vector vaccines improving immunization efficacy in poultry?
Recombinant vaccines, often utilizing HVT or Fowlpox vectors, improve efficacy by bypassing the interference caused by maternal antibodies in young chicks. They also typically provide long-lasting, stable immunity after a single dose and exhibit enhanced thermal stability compared to conventional live virus vaccines, simplifying administration logistics and improving protective coverage.
What role does Artificial Intelligence (AI) play in the future of the ND Vaccine market?
AI plays a pivotal role by enhancing precision and speed. AI is used for rapid analysis of viral genomics to detect mutations and update vaccine strains quickly, optimize manufacturing yields, and implement predictive modeling for localized outbreak forecasting, allowing for proactive, geographically targeted vaccine deployment and efficient supply chain management.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
- Newcastle Disease Vaccine Market Size Report By Type (Live Vaccines, Killed Vaccines), By Application (Chicken, Duck & Goose, Other), By Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Share, Trends, Outlook and Forecast 2025-2032
- Newcastle Disease Vaccine Market Size, Share, Trends, & Covid-19 Impact Analysis By Type (Live Vaccines, Killed Vaccines), By Application (Chicken, Duck & Goose, Other), By Region - North America, Latin America, Europe, Asia Pacific, Middle East, and Africa | In-depth Analysis of all factors and Forecast 2023-2030
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
