3D Printed Medical Devices & Implants Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 430122 | Date : Nov, 2025 | Pages : 242 | Region : Global | Publisher : MRU
3D Printed Medical Devices & Implants Market Size
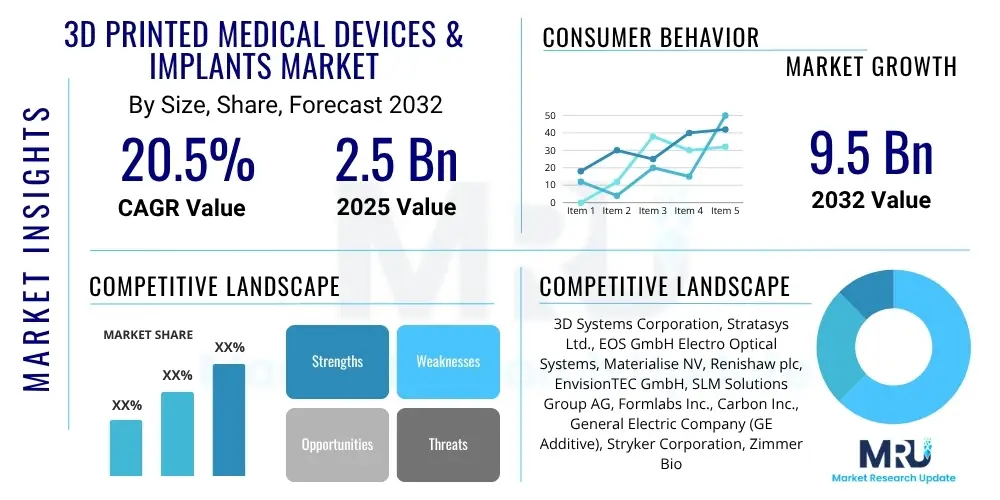
The 3D Printed Medical Devices & Implants Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 20.5% between 2025 and 2032. The market is estimated at USD 2.5 billion in 2025 and is projected to reach USD 9.5 billion by the end of the forecast period in 2032.
3D Printed Medical Devices & Implants Market introduction
The 3D Printed Medical Devices & Implants Market encompasses the design, production, and application of medical devices and implants using additive manufacturing techniques. This transformative technology allows for the creation of complex, patient-specific products directly from digital models, revolutionizing healthcare by offering unparalleled customization and precision. Products range from surgical guides, anatomical models for pre-operative planning, and prosthetic limbs to custom orthopedic implants, dental restorations, and even bioprinted tissues.
Major applications span across orthopedics, dentistry, cardiology, neurosurgery, and craniomaxillofacial surgery, where the ability to produce geometries impossible with traditional manufacturing methods is highly valued. The primary benefits include enhanced patient outcomes due to personalized fit, reduced surgical times, improved biocompatibility with advanced materials, and faster prototyping and product development cycles. This market is primarily driven by the increasing global prevalence of chronic diseases, a rapidly aging population requiring more implants, and continuous advancements in materials science and 3D printing technologies.
3D Printed Medical Devices & Implants Market Executive Summary
The 3D Printed Medical Devices & Implants Market is experiencing robust growth driven by technological innovation and the escalating demand for personalized healthcare solutions. Business trends indicate a significant increase in strategic partnerships, mergers and acquisitions among traditional medical device manufacturers and specialized 3D printing companies, aiming to consolidate expertise and expand market reach. Investments in research and development are also surging, particularly in advanced biocompatible materials and multi-material printing capabilities, alongside efforts to navigate and influence the evolving regulatory landscape.
Regionally, North America continues to dominate the market due to its advanced healthcare infrastructure, significant R&D spending, and early adoption of innovative medical technologies. Europe also holds a substantial share, backed by supportive government initiatives and a strong focus on patient-centric care. The Asia Pacific region is projected to exhibit the highest growth rate, fueled by improving healthcare access, rising disposable incomes, and increasing awareness of 3D printing benefits in countries like China, India, and Japan. Segment trends highlight the growing demand for orthopedic and dental implants, which currently constitute the largest application areas, with bioprinting and custom surgical instruments emerging as high-potential segments.
AI Impact Analysis on 3D Printed Medical Devices & Implants Market
User inquiries regarding AI's impact on 3D printed medical devices often center on how AI can accelerate design, improve material selection, personalize treatments further, and optimize manufacturing processes. The key themes revolve around AI's capacity to enhance precision, reduce development cycles, and unlock new possibilities for complex, functional medical products. Users anticipate AI will lead to more intelligent, responsive, and patient-specific devices, while also expressing interest in AI's role in quality control and regulatory compliance for these novel solutions.
- AI optimizes design parameters for patient-specific implants by analyzing vast datasets of medical images and patient anatomy.
- AI algorithms enhance material selection, predicting optimal material properties for specific biological applications and improving biocompatibility.
- Predictive analytics powered by AI contributes to the development of "smart" implants that monitor physiological conditions and provide real-time data.
- AI-driven simulation tools improve the accuracy of surgical planning and guide the printing of complex anatomical models and surgical guides.
- Generative design techniques, leveraging AI, create optimized structures for implants, leading to lighter, stronger, and more functional devices.
- AI aids in quality control and defect detection during the 3D printing process, ensuring the integrity and safety of medical devices.
- Machine learning models assist in identifying optimal printing parameters to reduce waste, improve efficiency, and ensure consistency in production.
DRO & Impact Forces Of 3D Printed Medical Devices & Implants Market
The 3D Printed Medical Devices & Implants Market is significantly influenced by a dynamic interplay of driving forces, inherent restraints, and burgeoning opportunities. Key drivers include the escalating global demand for personalized medicine, which 3D printing uniquely addresses through custom-fit solutions, and the continuous technological advancements in printing techniques and materials that expand the scope of applications. The rising incidence of orthopedic and dental conditions, coupled with a growing geriatric population, further fuels the need for innovative implant solutions, while increasing healthcare expenditure in emerging economies provides fertile ground for market expansion.
However, the market faces several significant restraints. The high initial capital investment required for 3D printing equipment, alongside the cost of specialized biocompatible materials, presents a barrier to entry for smaller players and limits adoption in less affluent regions. Stringent regulatory approval processes for novel 3D printed medical devices, coupled with intellectual property concerns around digital designs, also slow down market growth. Furthermore, the scarcity of skilled professionals proficient in both additive manufacturing and medical applications poses a challenge to widespread implementation and innovation.
Opportunities for growth are abundant, particularly in the realm of bioprinting for tissue engineering and regenerative medicine, which holds immense potential for future therapeutic applications. The development of advanced, multi-functional materials and the expansion of point-of-care manufacturing facilities offer avenues for market players to differentiate and innovate. Moreover, the increasing collaboration between academic institutions, research organizations, and industry players is expected to accelerate R&D, leading to new product introductions and broader market penetration.
Segmentation Analysis
The 3D Printed Medical Devices & Implants Market is comprehensively segmented based on various factors, including product type, material, application, and end-user. This segmentation provides a granular view of market dynamics, enabling stakeholders to understand specific growth drivers and emerging trends within each category. The product type segment typically differentiates between standard and custom implants, surgical instruments, and anatomical models, each serving distinct clinical needs. Material segmentation highlights the critical role of advanced polymers, metals, and ceramics in ensuring biocompatibility and functional performance.
Application-based segmentation showcases the diverse therapeutic areas benefiting from 3D printing, such as orthopedics, dentistry, and cardiology, each presenting unique demands and opportunities. Finally, the end-user analysis identifies the primary consumers of these devices, ranging from hospitals and ambulatory surgical centers to academic and research institutions. This multi-faceted segmentation underscores the broad applicability and evolving specialization within the 3D printed medical devices landscape, reflecting the market's maturity and future growth trajectories.
- By Product Type:
- Implants (Orthopedic, Dental, Cranio-maxillofacial, Spinal, Others)
- Surgical Instruments (Guides, Models, Tools)
- Prosthetics & Orthotics
- Bioprinted Tissues & Organs
- By Material:
- Polymers (PEEK, PLA, ABS, Nylon)
- Metals (Titanium, Stainless Steel, Cobalt-chrome)
- Ceramics (Hydroxyapatite, Zirconia)
- Composites
- Bio-inks
- By Application:
- Orthopedics
- Dental
- Cardiovascular
- Craniomaxillofacial
- Neurosurgery
- Others (e.g., General Surgery, Diagnostics)
- By End User:
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Dental Laboratories & Clinics
- Academic & Research Institutions
- Contract Manufacturing Organizations
- By Technology:
- Stereolithography (SLA)
- Fused Deposition Modeling (FDM)
- Selective Laser Sintering (SLS)
- Binder Jetting
- Material Jetting
- Digital Light Processing (DLP)
- Bioprinting
Value Chain Analysis For 3D Printed Medical Devices & Implants Market
The value chain for the 3D Printed Medical Devices & Implants Market is intricate, involving several distinct stages from raw material sourcing to end-user application. Upstream activities begin with raw material suppliers providing high-grade biocompatible polymers, metals, and ceramics, alongside specialized bio-inks. This stage also includes the development and provision of advanced 3D printing hardware (printers) and sophisticated software solutions, such as CAD/CAM tools, slicing software, and simulation platforms, which are critical for design and manufacturing precision. These foundational elements ensure the quality and capability of the subsequent production processes.
Midstream activities involve the actual manufacturing process, including the printing service providers, contract manufacturers specializing in medical 3D printing, and dedicated R&D facilities. This stage focuses on translating digital designs into physical devices, often requiring post-processing, sterilization, and rigorous quality control measures to meet medical standards. Downstream activities primarily involve distribution and end-user engagement. Distribution channels can be both direct, where manufacturers sell directly to hospitals or surgical centers, and indirect, involving specialized distributors who manage logistics and provide localized support. Sales and marketing efforts are crucial here to educate healthcare professionals about the benefits and applications of 3D printed medical devices.
The ultimate beneficiaries and key players in the downstream segment are healthcare providers, including hospitals, ambulatory surgical centers, and dental clinics, which integrate these devices into patient care. Academic and research institutions also play a vital role, not just as end-users for research purposes but also in driving innovation and validating new applications. The efficiency and transparency across all these stages are paramount, especially given the strict regulatory requirements and the need for precision and customization inherent in the medical device sector.
3D Printed Medical Devices & Implants Market Potential Customers
The primary potential customers and end-users for 3D Printed Medical Devices & Implants are diverse, reflecting the broad applicability of this technology across various healthcare settings. Hospitals and clinics represent a major segment, utilizing 3D printed anatomical models for pre-operative planning, custom surgical guides to enhance precision, and personalized implants for a wide range of specialties including orthopedics, neurosurgery, and cardiovascular procedures. The ability to offer patient-specific solutions directly impacts surgical success rates and recovery times, making these institutions key adopters.
Ambulatory Surgical Centers (ASCs) are increasingly investing in 3D printing capabilities, particularly for dental and orthopedic outpatient procedures, benefiting from faster turnaround times and reduced costs compared to traditional hospital settings. Dental laboratories and clinics are another significant customer base, driving demand for custom crowns, bridges, dentures, and orthodontic aligners, where the precision and speed of 3D printing are highly advantageous. Academic and research institutions serve as crucial customers, leveraging 3D printing for material science research, bioprinting of tissues, drug discovery models, and educational purposes, pushing the boundaries of what is medically possible.
Furthermore, contract manufacturing organizations (CMOs) that specialize in medical device production represent an indirect but vital customer segment, as they are often commissioned by larger medical device companies to produce 3D printed components or entire devices. These various end-user segments collectively define the demand landscape for the 3D Printed Medical Devices & Implants Market, each motivated by the unique benefits that additive manufacturing brings to their respective operations and patient care.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 2.5 billion |
| Market Forecast in 2032 | USD 9.5 billion |
| Growth Rate | 20.5% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | 3D Systems Corporation, Stratasys Ltd., EOS GmbH Electro Optical Systems, Materialise NV, Renishaw plc, EnvisionTEC GmbH, SLM Solutions Group AG, Formlabs Inc., Carbon Inc., General Electric Company (GE Additive), Stryker Corporation, Zimmer Biomet Holdings Inc., Smith & Nephew plc, Johnson & Johnson (DePuy Synthes), LimaCorporate S.p.A., OrthoPediatrics Corp., Bego GmbH & Co. KG, Dentsply Sirona Inc., Saremco Dental AG, Voxeljet AG |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
3D Printed Medical Devices & Implants Market Key Technology Landscape
The technology landscape for the 3D Printed Medical Devices & Implants Market is characterized by a diverse array of additive manufacturing processes, each offering unique capabilities for specific medical applications. Stereolithography (SLA) and Digital Light Processing (DLP) are widely used for their ability to produce high-resolution, intricate parts with smooth surfaces, ideal for dental applications, surgical guides, and anatomical models. These technologies utilize photopolymer resins, cured layer by layer by UV light, enabling precision and fine details critical in medical contexts.
Selective Laser Sintering (SLS) and Electron Beam Melting (EBM) are prominent for manufacturing metal implants, such as those made from titanium or cobalt-chrome. These powder bed fusion techniques produce strong, durable, and biocompatible parts with complex internal geometries, which are particularly advantageous for orthopedic and craniofacial implants that require high mechanical strength and osseointegration. Fused Deposition Modeling (FDM) offers a cost-effective solution for creating prototypes, surgical planning models, and certain non-critical medical devices using thermoplastic polymers.
Beyond these, material jetting technologies allow for multi-material printing and varying material properties within a single print, expanding possibilities for functional medical devices and multi-component prosthetics. Bioprinting, an advanced sub-segment, utilizes bio-inks containing living cells to create tissues and organs, representing the frontier of regenerative medicine. The continuous innovation in these printing technologies, coupled with advancements in medical-grade materials and sophisticated software for design and simulation, is driving the market forward, enabling the creation of increasingly complex, functional, and patient-specific medical solutions.
Regional Highlights
- North America: Dominates the market, driven by high healthcare expenditure, significant R&D investments, advanced technological adoption, and the presence of key market players and research institutions. The United States is a primary contributor to this dominance.
- Europe: Holds a substantial market share, supported by well-established healthcare infrastructure, favorable government initiatives promoting additive manufacturing in medicine, and a strong focus on personalized patient care in countries like Germany, the UK, and France.
- Asia Pacific (APAC): Expected to witness the highest CAGR during the forecast period. This growth is attributed to rising healthcare awareness, improving medical facilities, increasing government funding for medical device innovation, and a large patient pool in rapidly developing economies such as China, India, and Japan.
- Latin America: An emerging market with growing adoption of 3D printing technologies in healthcare. Countries like Brazil and Mexico are experiencing increased investments in healthcare infrastructure and medical tourism, contributing to market growth.
- Middle East and Africa (MEA): Shows nascent but promising growth, driven by increasing healthcare investments, a focus on medical innovation, and strategic partnerships with global leaders to enhance regional medical device manufacturing capabilities.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the 3D Printed Medical Devices & Implants Market.- 3D Systems Corporation
- Stratasys Ltd.
- EOS GmbH Electro Optical Systems
- Materialise NV
- Renishaw plc
- EnvisionTEC GmbH
- SLM Solutions Group AG
- Formlabs Inc.
- Carbon Inc.
- General Electric Company (GE Additive)
- Stryker Corporation
- Zimmer Biomet Holdings Inc.
- Smith & Nephew plc
- Johnson & Johnson (DePuy Synthes)
- LimaCorporate S.p.A.
- OrthoPediatrics Corp.
- Bego GmbH & Co. KG
- Dentsply Sirona Inc.
- Saremco Dental AG
- Voxeljet AG
Frequently Asked Questions
What are the primary benefits of 3D printed medical devices?
3D printed medical devices offer unparalleled patient-specific customization, leading to improved fit and enhanced surgical outcomes. They also enable the creation of complex geometries, reduce lead times for prototypes and final products, and support the use of advanced biocompatible materials, contributing to better patient care and efficiency.
What materials are commonly used in 3D printed medical implants?
Common materials include medical-grade polymers such as PEEK, PLA, and ABS; metals like titanium, stainless steel, and cobalt-chrome alloys; and ceramics such as hydroxyapatite and zirconia. Bio-inks containing living cells are also used in advanced bioprinting applications for tissue engineering.
What are the main applications of 3D printing in the medical sector?
Key applications include orthopedic implants (e.g., hip and knee replacements), dental restorations (crowns, bridges, aligners), craniomaxillofacial implants, surgical guides for precise operations, anatomical models for pre-operative planning and education, and custom prosthetics and orthotics.
What are the biggest challenges facing the 3D Printed Medical Devices & Implants Market?
Major challenges include the high cost of 3D printing equipment and specialized materials, stringent and evolving regulatory approval processes, the scarcity of skilled professionals proficient in both additive manufacturing and medical fields, and intellectual property concerns related to digital designs and manufacturing files.
How is artificial intelligence (AI) impacting 3D printed medical devices?
AI significantly impacts the market by optimizing device design, enhancing material selection, personalizing implants based on patient data, and improving manufacturing efficiency through predictive analytics. It also aids in quality control and accelerates research and development, leading to more intelligent and functional medical devices.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
