Acitretin Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429774 | Date : Nov, 2025 | Pages : 253 | Region : Global | Publisher : MRU
Acitretin Market Size
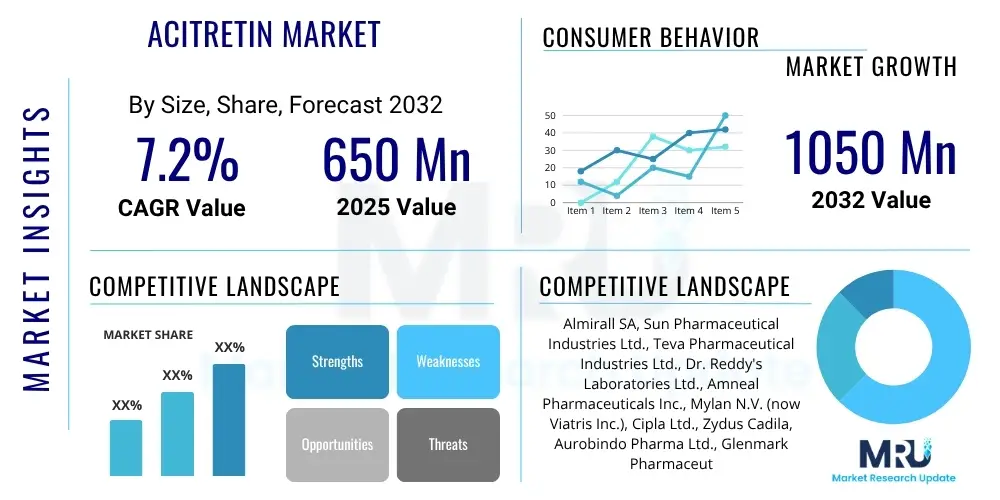
The Acitretin Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.2% between 2025 and 2032. The market is estimated at USD 650 Million in 2025 and is projected to reach USD 1050 Million by the end of the forecast period in 2032.
Acitretin Market introduction
The Acitretin market encompasses pharmaceutical products containing acitretin, a synthetic retinoid derived from vitamin A, primarily utilized for the systemic treatment of severe and recalcitrant forms of psoriasis and other disorders of keratinization. This medication plays a crucial role in dermatology by regulating cell growth and differentiation, thereby slowing down the excessive skin cell production characteristic of conditions like psoriasis. The market is defined by its application in addressing chronic and debilitating dermatological conditions where topical treatments or phototherapy have proven insufficient or contraindicated, offering a systemic approach to disease management.
Acitretin is typically prescribed for severe forms of psoriasis, including erythrodermic psoriasis, pustular psoriasis, and severe generalized plaque psoriasis, particularly when other systemic therapies are not suitable or effective. It is also indicated for congenital ichthyosis and other severe disorders where accelerated or abnormal keratinization is the underlying pathology. Its mechanism involves binding to nuclear retinoid receptors, which subsequently alters gene expression involved in epidermal proliferation and differentiation, ultimately leading to a normalization of skin cell turnover. The primary benefits of Acitretin include its potent efficacy in clearing severe psoriatic lesions, reducing inflammation, and improving quality of life for patients who experience significant physical and psychological burden from their condition.
The market's growth is predominantly driven by the increasing global prevalence of chronic dermatological conditions such as psoriasis, coupled with a rising awareness among both patients and healthcare providers regarding advanced systemic treatment options. Furthermore, an aging population, which is more susceptible to various chronic diseases including psoriasis, contributes significantly to the demand for effective therapies. Advancements in diagnostic techniques leading to earlier and more accurate identification of severe cases also expand the eligible patient pool for Acitretin, solidifying its position as a vital therapeutic agent in dermatological practice.
Acitretin Market Executive Summary
The Acitretin market is poised for steady expansion, propelled by persistent demand for effective treatments for severe psoriasis and other keratinization disorders. Business trends indicate a continuous focus on generic drug development to improve accessibility and affordability, alongside ongoing research into safer retinoid derivatives and combination therapies to enhance efficacy and mitigate side effects. Pharmaceutical companies are increasingly exploring market penetration strategies in underserved regions, recognizing the growing prevalence of dermatological conditions in these areas. There is also a rising emphasis on patient education and adherence programs, crucial for managing a medication with a significant side effect profile like Acitretin.
Regionally, mature markets in North America and Europe continue to represent significant revenue streams, characterized by established healthcare infrastructures, high disease awareness, and strong prescribing patterns for systemic dermatological drugs. However, the most dynamic growth is anticipated from emerging economies in Asia Pacific and Latin America. These regions are experiencing rapid improvements in healthcare access, increasing disposable incomes, and a rising diagnosis rate of chronic skin conditions, thereby creating substantial opportunities for market expansion. Government initiatives to improve healthcare infrastructure and a growing number of dermatologists in these areas further contribute to the favorable regional outlook for Acitretin.
From a segmentation perspective, the oral capsule formulation of Acitretin remains the dominant product type, reflecting its systemic action and established efficacy for severe conditions. Application-wise, psoriasis continues to be the primary driver, though its use in other rare keratinization disorders contributes to market stability. The distribution channels are evolving, with hospital pharmacies and specialized dermatology clinics maintaining their critical role in initial prescription and dispensing, while retail and online pharmacies are gaining traction for prescription refills and enhanced patient convenience, especially as telemedicine adoption increases. This diversification of distribution pathways supports broader patient access to Acitretin, even amidst the need for careful patient monitoring.
AI Impact Analysis on Acitretin Market
The integration of Artificial Intelligence (AI) holds transformative potential for the Acitretin market, addressing critical user questions related to personalized treatment, adverse event prediction, and drug development efficiency. Users are keenly interested in how AI can help tailor Acitretin dosages to individual patient profiles, minimizing severe side effects while maximizing therapeutic outcomes, particularly given the drug's teratogenicity and hepatotoxicity. There is also significant curiosity about AI's role in identifying suitable patient candidates who would most benefit from Acitretin versus alternative treatments, moving beyond broad diagnostic categories. Furthermore, the market anticipates AI's contribution to accelerating research into safer retinoid analogs or novel combination therapies that could potentially reduce the current limitations of Acitretin, thereby enhancing its market viability and patient adoption rates.
- AI-driven patient stratification to identify optimal responders to Acitretin, improving treatment efficacy.
- Predictive analytics for early detection and management of Acitretin's adverse effects, enhancing patient safety.
- Personalized dosage recommendations and treatment plans based on genetic markers and real-world patient data.
- Accelerated drug discovery and development of next-generation retinoids with improved safety profiles using AI algorithms.
- Enhanced pharmacovigilance through AI-powered monitoring of real-world evidence for drug safety surveillance.
- Streamlined clinical trial design and patient recruitment for new retinoid-based therapies.
- Optimized supply chain and inventory management for Acitretin, ensuring consistent availability.
- AI-assisted diagnostic tools for more accurate and early identification of severe psoriasis and related conditions.
- Development of AI models to understand patient adherence patterns and improve medication persistence for Acitretin.
- Identification of novel therapeutic targets and pathways related to keratinization disorders.
DRO & Impact Forces Of Acitretin Market
The Acitretin market is significantly influenced by a complex interplay of drivers, restraints, and opportunities that shape its growth trajectory and competitive landscape. A primary driver is the increasing global prevalence of severe dermatological conditions such as psoriasis, which necessitates effective systemic treatments. As populations age and lifestyle factors contribute to the onset and exacerbation of chronic skin diseases, the demand for drugs like Acitretin remains robust. Furthermore, rising healthcare expenditure, improved diagnostic capabilities, and growing awareness among patients and healthcare professionals about various treatment modalities contribute to a broader acceptance and adoption of systemic retinoids, solidifying their market presence. The consistent need for therapies in cases unresponsive to or intolerant of other treatments underscores Acitretin's critical role.
Conversely, the market faces notable restraints, most prominently the severe side effect profile of Acitretin, including its potent teratogenicity, which necessitates strict contraindications for women of childbearing potential, and potential hepatotoxicity, requiring regular liver function monitoring. These safety concerns lead to stringent regulatory guidelines and require extensive patient counseling, often limiting its prescription. The emergence of biologics and small molecule inhibitors as highly effective, and often better-tolerated, alternatives for severe psoriasis presents significant competition, potentially diverting patients away from traditional systemic retinoids. Additionally, the high cost associated with branded Acitretin, despite the availability of generics, can pose an access barrier in certain healthcare systems, impacting market penetration.
Amidst these challenges, significant opportunities exist for market expansion and innovation. Research and development into novel Acitretin formulations or safer retinoid derivatives that retain efficacy while minimizing adverse effects could revitalize the market. Exploring combination therapies, where Acitretin is used synergistically with other treatments to achieve better outcomes at lower individual doses, also presents a promising avenue. The expansion of healthcare infrastructure and increasing access to specialized dermatological care in emerging economies offer untapped patient populations. Additionally, the application of telemedicine for remote patient monitoring and follow-up can improve adherence and safety surveillance for patients on Acitretin, enhancing its overall management and potentially broadening its utility in areas with limited specialist access. These opportunities, if effectively leveraged, can help overcome existing market hurdles and drive future growth.
Segmentation Analysis
The Acitretin market is comprehensively segmented to provide a detailed understanding of its various facets, allowing for precise market analysis and strategic planning. These segmentations are crucial for identifying key trends, understanding demand patterns, and evaluating growth opportunities across different dimensions. The primary segmentation categories include the application of Acitretin, the type of dosage form, and the distribution channels through which the product reaches end-users. Each segment offers distinct insights into patient needs, treatment preferences, and market dynamics, reflecting the diverse landscape of dermatological care. Understanding these segments is vital for pharmaceutical companies to tailor their product development, marketing, and sales strategies effectively. For instance, analyzing the application segment helps identify the most prevalent conditions driving Acitretin demand, while dosage form analysis highlights product preference, and distribution channel analysis indicates the most efficient ways to reach patients.
- By Application:
- Psoriasis:
- Severe Psoriasis Vulgaris
- Erythrodermic Psoriasis
- Pustular Psoriasis
- Ichthyosis:
- Congenital Ichthyosis
- Other Severe Ichthyoses
- Other Keratinization Disorders:
- Lichen Planus
- Darier's Disease
- Palmoplantar Keratodermas
- Psoriasis:
- By Dosage Form:
- Oral Capsules:
- 10 mg Capsules
- 25 mg Capsules
- Oral Capsules:
- By Distribution Channel:
- Hospital Pharmacies:
- In-patient Dispensing
- Out-patient Dispensing
- Retail Pharmacies:
- Community Pharmacies
- Chain Drugstores
- Online Pharmacies:
- E-commerce Pharmaceutical Platforms
- Telepharmacy Services
- Hospital Pharmacies:
- By End-User:
- Hospitals:
- Dermatology Departments
- General Medicine Departments
- Dermatology Clinics:
- Specialized Skin Care Centers
- Private Practice Clinics
- Homecare Settings:
- Patient Self-Administration
- Remote Monitoring
- Hospitals:
- By Regional Outlook:
- North America (U.S., Canada)
- Europe (Germany, U.K., France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, Japan, India, South Korea, Rest of Asia Pacific)
- Latin America (Brazil, Mexico, Rest of Latin America)
- Middle East & Africa (GCC Countries, South Africa, Rest of MEA)
Value Chain Analysis For Acitretin Market
The value chain for the Acitretin market begins with the upstream activities involving research and development, active pharmaceutical ingredient (API) manufacturing, and raw material sourcing. This initial phase is highly complex and regulated, focusing on the synthesis of acitretin, its purification, and the procurement of excipients required for formulation. Key players in this segment include specialized chemical manufacturers and pharmaceutical companies with extensive R&D capabilities. Upstream activities are critical for ensuring the quality, purity, and consistent supply of the active ingredient, which directly impacts the safety and efficacy of the final pharmaceutical product. Regulatory compliance, intellectual property protection, and efficient synthesis processes are paramount at this stage to establish a strong foundation for the entire value chain.
Following the upstream processes, the value chain progresses to the core manufacturing and formulation of Acitretin into its final dosage form, typically oral capsules. This stage involves sophisticated pharmaceutical manufacturing processes, quality control, and packaging. After manufacturing, the products enter the downstream segment, which includes logistics, distribution, marketing, and sales. The distribution channel is multifaceted, comprising direct sales to large hospital groups, indirect distribution through wholesalers to retail pharmacies and smaller clinics, and increasingly, online pharmacies facilitating broader patient access. Effective logistics and supply chain management are essential to ensure timely delivery and minimize stock-outs, particularly given the specialized nature and controlled distribution of Acitretin due to its safety profile.
The final stages of the value chain involve the prescribing healthcare professionals, pharmacists, and ultimately, the patients. Direct channels involve manufacturers engaging directly with key opinion leaders, dermatologists, and large hospital networks for product promotion and education. Indirect channels primarily involve pharmaceutical wholesalers and distributors who serve as intermediaries, transporting Acitretin from manufacturing facilities to a wide network of retail pharmacies, hospital pharmacies, and specialized dermatology clinics. This ensures broad availability while adhering to the necessary cold chain and storage requirements. Patient education and adherence programs, often facilitated by healthcare providers and pharmacies, represent the concluding part of the value chain, ensuring safe and effective use of the medication and fostering positive patient outcomes, which in turn influences market demand and growth.
Acitretin Market Potential Customers
The primary potential customers and end-users of Acitretin are individuals suffering from severe, chronic, and recalcitrant forms of psoriasis and other severe disorders of keratinization that have not responded adequately to alternative treatments. This includes patients with erythrodermic psoriasis, generalized pustular psoriasis, and severe plaque psoriasis covering a significant body surface area, where topical therapies or phototherapy have been ineffective or are contraindicated. Patients diagnosed with congenital ichthyosis and other rare genetic disorders characterized by abnormal skin keratinization also represent a crucial segment of the customer base. These patients often face significant challenges to their quality of life due to their skin conditions, making them actively seek highly effective systemic treatments.
In addition to patients, the professional buyers and prescribers are key customers in the Acitretin market. This includes dermatologists who specialize in managing severe skin conditions and are responsible for diagnosing and prescribing Acitretin based on clinical guidelines and patient profiles. Hospitals, particularly those with specialized dermatology departments, and private dermatology clinics are significant organizational buyers, as they stock and dispense Acitretin to their patient populations. Moreover, pharmacists in hospital, retail, and online settings play a vital role in dispensing the medication, providing patient counseling, and ensuring adherence to safety protocols, particularly concerning the teratogenic risks of Acitretin. These healthcare providers and institutions are crucial intermediaries that influence patient access and utilization of the product.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 650 Million |
| Market Forecast in 2032 | USD 1050 Million |
| Growth Rate | 7.2% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Almirall SA, Sun Pharmaceutical Industries Ltd., Teva Pharmaceutical Industries Ltd., Dr. Reddy's Laboratories Ltd., Amneal Pharmaceuticals Inc., Mylan N.V. (now Viatris Inc.), Cipla Ltd., Zydus Cadila, Aurobindo Pharma Ltd., Glenmark Pharmaceuticals Ltd., Intas Pharmaceuticals Ltd., Torrent Pharmaceuticals Ltd., Lupin Ltd., Novartis AG, Leo Pharma A/S, AbbVie Inc., Johnson & Johnson, Pfizer Inc., Merck & Co. Inc., Bristol-Myers Squibb Company |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Acitretin Market Key Technology Landscape
The technological landscape surrounding the Acitretin market is primarily focused on optimizing drug formulation, improving drug delivery systems, and enhancing patient safety and adherence through innovative healthcare technologies. While Acitretin itself is an established molecule, ongoing advancements in pharmaceutical technology aim to refine its therapeutic utility. This includes the development of more stable and bioavailable formulations that could potentially reduce variability in patient response or allow for lower effective doses, thereby mitigating some of its dose-dependent side effects. Research into alternative delivery mechanisms, though challenging for a systemic retinoid, also represents an area of potential technological advancement, exploring methods to provide more localized or controlled release, if applicable to broader retinoid therapy.
Furthermore, advancements in pharmacogenomics and personalized medicine are becoming increasingly important. These technologies enable a deeper understanding of how individual genetic variations might influence a patient's response to Acitretin, including its efficacy and propensity for adverse reactions. By leveraging genetic markers, healthcare providers can potentially identify patients who are more likely to benefit from Acitretin or those who might be at a higher risk of severe side effects, leading to more tailored and safer treatment regimens. This personalized approach aims to optimize therapeutic outcomes while minimizing risks, a critical consideration for a drug with a narrow therapeutic window and significant safety concerns.
Beyond drug-specific technologies, broader healthcare innovations, particularly in digital health and data analytics, significantly impact the Acitretin market. Telemedicine platforms and remote patient monitoring devices are crucial for enhancing patient follow-up and adverse event surveillance, which is essential for Acitretin therapy due to its requirement for regular blood tests and pregnancy precautions. Electronic health records (EHRs) integrated with clinical decision support systems can assist prescribers in adhering to strict prescribing guidelines and monitoring parameters. Advanced analytical techniques, including AI and machine learning, are increasingly employed in pharmacovigilance to analyze real-world data, identify emerging safety signals, and improve risk-benefit assessments for Acitretin and similar drugs. These technological advancements collectively contribute to safer and more effective management of patients receiving Acitretin therapy.
Regional Highlights
- North America: This region, particularly the United States and Canada, represents a significant market share due to a high prevalence of psoriasis, advanced healthcare infrastructure, high awareness among patients and healthcare providers, and robust reimbursement policies. The presence of major pharmaceutical companies and active research and development initiatives further contributes to market growth. Strong regulatory frameworks ensure drug quality and safety, fostering patient and physician confidence in prescribed treatments.
- Europe: Countries like Germany, the UK, France, Italy, and Spain are key contributors to the Acitretin market in Europe. The region benefits from well-established healthcare systems, a substantial patient pool with chronic dermatological conditions, and a proactive approach to patient care. Strict regulations by bodies like the European Medicines Agency (EMA) guide drug use, while ongoing clinical research and a focus on improving patient quality of life sustain market demand.
- Asia Pacific (APAC): The APAC region, encompassing countries such as China, Japan, India, and South Korea, is projected to witness the fastest growth. This surge is attributed to improving healthcare access, increasing disposable incomes, a growing number of dermatologists, and rising awareness of chronic skin diseases. The large and aging population, coupled with increasing urbanization and lifestyle changes, contributes to a higher incidence of dermatological conditions, fueling demand for effective treatments like Acitretin.
- Latin America: The market in Latin American countries, including Brazil and Mexico, is experiencing steady growth driven by expanding healthcare infrastructure, increasing government expenditure on public health, and a rising awareness of dermatological health. Economic development and greater access to medical services are enabling more patients to seek diagnosis and treatment for severe psoriasis, thereby contributing to the demand for Acitretin.
- Middle East and Africa (MEA): This region presents emerging opportunities for the Acitretin market. While currently smaller in market size compared to other regions, MEA is characterized by developing healthcare systems, increasing investment in medical facilities, and a growing recognition of the need for specialized dermatological care. The increasing prevalence of chronic diseases and efforts to improve healthcare accessibility are expected to drive gradual growth in the coming years.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Acitretin Market.- Almirall SA
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Dr. Reddy's Laboratories Ltd.
- Amneal Pharmaceuticals Inc.
- Mylan N.V. (now Viatris Inc.)
- Cipla Ltd.
- Zydus Cadila
- Aurobindo Pharma Ltd.
- Glenmark Pharmaceuticals Ltd.
- Intas Pharmaceuticals Ltd.
- Torrent Pharmaceuticals Ltd.
- Lupin Ltd.
- Novartis AG
- Leo Pharma A/S
- AbbVie Inc.
- Johnson & Johnson
- Pfizer Inc.
- Merck & Co. Inc.
- Bristol-Myers Squibb Company
Frequently Asked Questions
What is Acitretin primarily used for?
Acitretin is primarily used for the systemic treatment of severe, recalcitrant psoriasis, including erythrodermic, pustular, and severe plaque psoriasis. It is also indicated for other severe disorders of keratinization, such as congenital ichthyosis, where other therapies have been ineffective or are contraindicated.
What are the major side effects and contraindications of Acitretin?
Major side effects include mucocutaneous dryness (lips, nose, eyes), hair loss, peeling skin, and liver function abnormalities. The most critical contraindication is its severe teratogenicity, meaning it can cause severe birth defects, requiring strict pregnancy prevention for women of childbearing potential during and for up to three years after treatment.
How does Acitretin compare to biologics for psoriasis treatment?
Acitretin is a traditional systemic retinoid, effective but with a significant side effect profile. Biologics are newer, highly targeted therapies that often have a more favorable safety profile and can be more potent for some severe psoriasis cases. Acitretin is often considered before biologics or in combination, depending on patient factors, disease severity, and comorbidities.
Is Acitretin safe for long-term use, and what monitoring is required?
Long-term use of Acitretin requires careful consideration due to potential cumulative toxicity, particularly affecting the liver and lipids. Patients require regular monitoring of liver function tests, lipid profiles, and blood counts. For women, strict pregnancy tests and contraception are mandatory throughout and for an extended period after treatment.
What are the future prospects for the Acitretin market?
The Acitretin market is expected to see stable demand due to the increasing prevalence of psoriasis. Future prospects include advancements in personalized medicine for patient selection, research into safer retinoid derivatives, and expanded access in emerging markets. Digital health tools for monitoring and adherence may also enhance its safe and effective use.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
