AI in Clinical Trials Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427756 | Date : Oct, 2025 | Pages : 245 | Region : Global | Publisher : MRU
AI in Clinical Trials Market Size
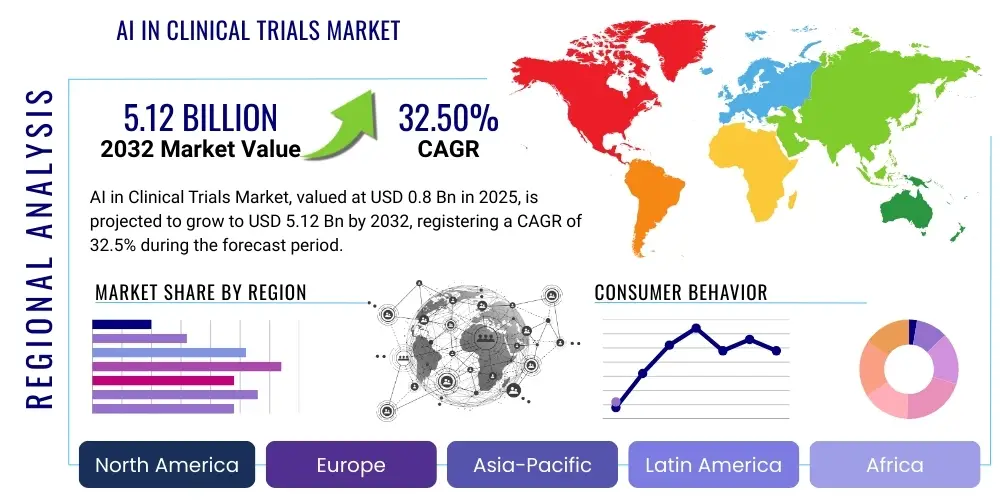
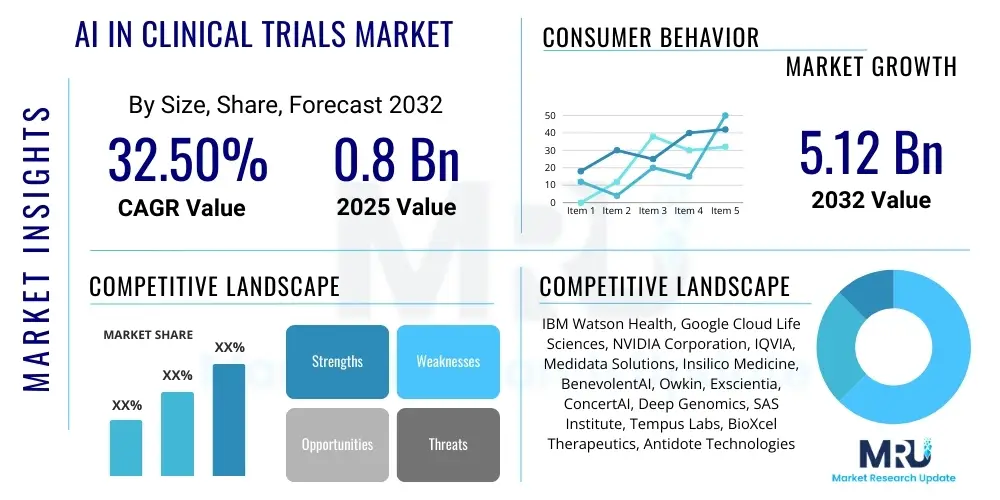
The AI in Clinical Trials Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 32.5% between 2025 and 2032. The market is estimated at USD 0.8 Billion in 2025 and is projected to reach USD 5.12 Billion by the end of the forecast period in 2032.
AI in Clinical Trials Market introduction
The AI in Clinical Trials Market is experiencing transformative growth, driven by the imperative to accelerate drug discovery, reduce development costs, and enhance the efficacy of new therapies. Artificial intelligence encompasses a range of sophisticated technologies, including machine learning, natural language processing, and predictive analytics, which are increasingly being deployed across various stages of clinical research. These AI solutions are designed to optimize trial design, streamline patient recruitment and stratification, improve data management and analysis, and predict clinical outcomes with greater accuracy. Major applications span from early drug target identification and lead optimization to real-world evidence generation and post-market surveillance. The primary benefits include significant reductions in trial timelines, lower operational expenditures, improved success rates for drug candidates, and the potential for more personalized and effective treatments. Key driving factors propelling this market include the escalating volume of complex biomedical data, the rising demand for precision medicine, the high failure rates and substantial costs associated with traditional clinical trials, and growing regulatory support for innovative technologies in healthcare.
AI in Clinical Trials Market Executive Summary
The AI in Clinical Trials Market is characterized by robust innovation and strategic collaborations aimed at integrating advanced computational capabilities into the drug development pipeline. Business trends indicate a strong emphasis on developing specialized AI platforms tailored for specific clinical trial phases, alongside a growing move towards cloud-based AI solutions that offer scalability and accessibility. There is an increasing prevalence of partnerships between AI technology providers, pharmaceutical companies, and contract research organizations (CROs) to co-develop and deploy AI-driven tools. Regionally, North America maintains its leadership position, fueled by substantial R&D investments, a mature technological infrastructure, and a supportive regulatory environment. Europe is demonstrating significant growth, driven by national AI strategies and established research hubs, while the Asia Pacific region is emerging as a critical growth engine, attributed to its large patient populations, increasing healthcare expenditure, and expanding digital infrastructure. Segmentation trends highlight the dominance of software solutions, particularly those focused on data management and patient selection, and a strong uptake across late-stage clinical trials where large datasets are generated. The market is also seeing differentiation by end-user, with pharmaceutical and biotechnology companies being the primary adopters, closely followed by CROs leveraging AI to enhance their service offerings and competitive edge.
AI Impact Analysis on AI in Clinical Trials Market
Users frequently inquire about how Artificial Intelligence truly transforms clinical trials, focusing on its ability to accelerate drug development, enhance success rates, and personalize treatment. Common questions revolve around the ethical implications of AI in patient data handling, the accuracy and validation of AI algorithms, and the practical challenges of integrating these technologies into existing workflows. There is significant interest in understanding AIs role in identifying suitable patient cohorts, optimizing trial protocols, and predicting adverse events, alongside concerns regarding data bias, the need for human oversight, and the high initial investment required for AI implementation. Users seek clarity on how AI can democratize access to trials and reduce the overall cost burden, while also questioning the readiness of the regulatory landscape to accommodate such rapid technological advancements.
- Accelerated Drug Discovery: AI algorithms analyze vast datasets, identifying potential drug candidates and optimal targets much faster than traditional methods.
- Enhanced Patient Recruitment and Stratification: AI uses predictive analytics to identify eligible patients, improving enrollment efficiency and ensuring more homogeneous study groups.
- Optimized Clinical Trial Design: AI tools simulate trial outcomes, suggest optimal dosing, and refine protocol design, leading to more efficient and less costly trials.
- Improved Data Management and Analysis: AI automates data collection, cleaning, and analysis, reducing human error and uncovering insights quickly from complex datasets.
- Predictive Biomarker Identification: AI assists in identifying novel biomarkers for disease progression and treatment response, enabling precision medicine approaches.
- Reduced Trial Costs and Duration: By streamlining multiple stages, AI significantly lowers the overall expenses and time required for clinical development.
- Personalized Medicine Advancement: AI facilitates the development of therapies tailored to individual patient profiles, moving towards more effective and targeted treatments.
- Early Detection of Adverse Events: AI monitors real-time patient data to identify safety signals and potential adverse drug reactions sooner.
- Real-World Evidence Generation: AI analyzes real-world data from electronic health records and wearables, providing valuable insights beyond traditional trial settings.
- Increased Trial Success Rates: By optimizing design and patient selection, AI contributes to a higher likelihood of successful trial outcomes.
DRO & Impact Forces Of AI in Clinical Trials Market
The AI in Clinical Trials Market is profoundly shaped by a confluence of driving factors, persistent restraints, and significant opportunities, all underscored by various impact forces that influence market dynamics. Key drivers include the exponential growth in biomedical data, which AI is uniquely positioned to process and derive insights from, as well as the escalating demand for personalized medicine approaches that require sophisticated analytical capabilities. The high costs and historically low success rates associated with traditional clinical trials further incentivize the adoption of AI to improve efficiency and mitigate risks. Conversely, the market faces considerable restraints such as stringent data privacy regulations, particularly concerning sensitive patient information, and the inherent challenges in validating complex AI algorithms for clinical use. A significant hurdle also lies in the scarcity of skilled personnel proficient in both clinical research and AI, alongside the complexities of integrating new AI systems with legacy IT infrastructures. However, numerous opportunities exist, including the expansion into untapped therapeutic areas, the potential for AI-driven drug repurposing, and the integration of real-world evidence into regulatory submissions. These factors create a dynamic environment where innovation is balanced against the need for rigorous validation and ethical considerations.
The impact forces within the AI in Clinical Trials Market are multifaceted, influencing strategic decisions and competitive landscapes. The bargaining power of buyers, primarily pharmaceutical and biotechnology companies, is moderate to high as they demand robust, validated, and cost-effective AI solutions that deliver tangible improvements in trial outcomes. The bargaining power of suppliers, consisting of specialized AI solution providers and cloud service platforms, is also significant due to their proprietary technologies and expertise, though increased competition is gradually tempering this. The threat of new entrants is moderate, as while the demand for AI in trials is high, developing and validating clinically relevant AI solutions requires substantial R&D investment, deep domain expertise, and navigating complex regulatory pathways. However, tech giants entering healthcare could lower this barrier. The threat of substitute products or technologies is relatively low, as conventional clinical trial methods are increasingly proving insufficient for complex modern drug development, and AI offers unique capabilities that are difficult to replicate. Finally, competitive rivalry among existing players is intense, characterized by continuous innovation, strategic partnerships, and a focus on demonstrating clear return on investment to gain market share. These forces collectively shape the markets evolution, pushing companies towards differentiation and specialized offerings.
Segmentation Analysis
The AI in Clinical Trials market is comprehensively segmented to provide granular insights into its diverse applications, technological underpinnings, and end-user adoption patterns. This segmentation allows for a detailed understanding of market dynamics across various components, including software and services, and by the specific applications where AI brings the most value, such as patient recruitment, data management, and drug discovery. Further divisions are made based on the clinical trial phase (Phase I, II, III, IV) where AI is deployed, reflecting the varying needs and data complexities at each stage. End-user segmentation highlights the primary beneficiaries and adopters of these technologies, including pharmaceutical companies, contract research organizations (CROs), and academic research institutions. This multi-dimensional approach to segmentation underscores the broad utility and specialized nature of AI solutions within the clinical trial ecosystem, providing clarity on growth areas and competitive landscapes for stakeholders.
- By Component:
- Software
- Services
- By Application:
- Drug Discovery
- Patient Recruitment and Retention
- Trial Design and Optimization
- Data Management and Analysis
- Drug Repurposing
- Predictive Analytics for Outcomes
- Post-Market Surveillance
- By Clinical Trial Phase:
- Phase I
- Phase II
- Phase III
- Phase IV
- By End User:
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic & Research Institutions
- Medical Device Companies
- By Technology:
- Machine Learning (Deep Learning, NLP)
- Computer Vision
- Predictive Analytics
- Robotics and Automation
AI in Clinical Trials Market Value Chain Analysis
The value chain for the AI in Clinical Trials Market commences with upstream activities focused on foundational research and technological development. This involves AI model development, often leveraging advanced machine learning, natural language processing, and predictive analytics techniques, alongside the crucial process of data preprocessing and curation. Upstream providers include specialized AI software developers, data scientists, and cloud infrastructure providers who supply the core computational power and algorithms. These entities are responsible for creating the sophisticated tools and platforms that form the bedrock of AI applications in clinical research. The quality and robustness of these upstream contributions directly influence the effectiveness and reliability of downstream clinical trial processes, emphasizing the importance of rigorous validation and continuous innovation in AI methodologies. Effective upstream collaboration ensures that AI solutions are built on sound scientific principles and are capable of handling the complexities of biological and clinical data. Companies specializing in machine learning frameworks, data annotation services, and high-performance computing are critical components at this initial stage, laying the groundwork for clinical utility.
Moving downstream, the value chain progresses to the application and utilization of these AI technologies within the clinical trial environment. This stage is primarily dominated by clinical trial sponsors, including pharmaceutical and biotechnology companies, as well as Contract Research Organizations (CROs), which integrate AI tools into their research pipelines. These downstream users leverage AI for various purposes, such as optimizing trial design, identifying and recruiting eligible patients, managing and analyzing vast amounts of clinical data, and predicting trial outcomes. The distribution channel for AI solutions can be direct, with AI technology providers selling software licenses or offering services directly to pharma companies and CROs. Alternatively, indirect channels involve partnerships, platform integrations (e.g., through cloud marketplaces), or collaborations with broader clinical research service providers. The ultimate beneficiaries are patients, who gain access to safer and more effective treatments more rapidly, and the healthcare system, which benefits from increased efficiency and reduced development costs. The interplay between upstream innovation and downstream adoption determines the overall market impact and the realization of AIs full potential in accelerating drug development and improving patient care outcomes.
AI in Clinical Trials Market Potential Customers
The primary potential customers and end-users of AI in Clinical Trials solutions are entities deeply involved in the development and validation of new medical interventions. Pharmaceutical and Biotechnology Companies represent the largest segment, as they are continually striving to reduce the exorbitant costs and extended timelines associated with traditional drug development, making AI a strategic imperative for competitive advantage and accelerated market entry. These companies utilize AI across all phases of drug discovery and clinical trials, from target identification to post-market surveillance, to enhance efficiency and decision-making. Contract Research Organizations (CROs) also constitute a significant customer base, adopting AI tools to augment their service offerings, streamline operations, and provide more sophisticated data analysis capabilities to their clients. By integrating AI, CROs can offer faster, more accurate, and cost-effective services, thereby attracting more business and differentiating themselves in a competitive landscape. Additionally, Academic and Research Institutions are key customers, leveraging AI for fundamental research, hypothesis generation, and the exploration of new therapeutic avenues. These institutions often engage in early-stage research and clinical investigations, where AI can significantly enhance the speed and scope of scientific discovery. The adoption by these diverse customer groups reflects a broad recognition of AIs potential to revolutionize the entire clinical research ecosystem, driving innovation and ultimately benefiting patient outcomes across various therapeutic areas.
AI in Clinical Trials Market Key Technology Landscape
The AI in Clinical Trials market is underpinned by a diverse and rapidly evolving technological landscape, with several key technologies driving innovation and efficacy. Machine Learning (ML), encompassing deep learning and natural language processing (NLP), forms the core of many AI applications. Deep learning algorithms are particularly adept at recognizing complex patterns in vast, unstructured datasets such as medical images, genomic sequences, and electronic health records, enabling precise patient stratification and biomarker discovery. Natural language processing, on the other hand, is crucial for extracting meaningful insights from unstructured text data, including scientific literature, clinical notes, and patient-reported outcomes, significantly accelerating information retrieval and synthesis for trial design and adverse event monitoring. These ML capabilities are instrumental in automating repetitive tasks, predicting outcomes, and identifying subtle relationships within complex biological systems, which would be impossible for human analysis alone.
Beyond core machine learning, predictive analytics plays a pivotal role, utilizing statistical algorithms and historical data to forecast future events, such as patient enrollment rates, potential safety issues, and trial success probabilities. This foresight allows for proactive adjustments to trial protocols and resource allocation. Computer Vision technologies are increasingly important for analyzing medical images (e.g., MRI, CT scans, pathology slides) to identify disease progression, evaluate treatment response, and detect subtle abnormalities that may serve as clinical endpoints. Furthermore, the immense data generation in clinical trials necessitates robust Big Data Analytics platforms for efficient storage, processing, and interpretation of heterogeneous data sources. Cloud Computing provides the scalable infrastructure required to handle these massive datasets and run complex AI models, offering flexibility and accessibility to research teams globally. Emerging technologies like Blockchain are also being explored for enhancing data integrity, security, and traceability within clinical trial processes, building trust and ensuring regulatory compliance. The synergistic application of these technologies is collectively driving the transformation of clinical research, making trials more intelligent, efficient, and ultimately more successful in bringing new treatments to patients.
Regional Highlights
- North America: This region consistently dominates the AI in Clinical Trials Market, primarily due to significant R&D investments by pharmaceutical giants and technology companies, a robust ecosystem of AI startups, and a strong emphasis on precision medicine. The presence of leading academic research institutions, advanced healthcare infrastructure, and a proactive regulatory environment further contribute to its market leadership. High adoption rates of cutting-edge technologies and a large pool of skilled professionals also propel growth.
- Europe: Europe represents a rapidly growing market, driven by increasing government funding for AI research, a strong academic-industrial collaboration framework, and a focus on digital health initiatives. Countries like the UK, Germany, and France are at the forefront, leveraging strong bioinformatics capabilities and supportive regulatory bodies (e.g., EMA) to integrate AI into clinical development. Data privacy concerns, however, necessitate careful implementation strategies under GDPR.
- Asia Pacific: The Asia Pacific region is emerging as a high-growth market, propelled by large and diverse patient populations, increasing healthcare expenditure, and a growing focus on pharmaceutical R&D in countries like China, India, and Japan. Governments are actively promoting AI adoption in healthcare, and the region offers a significant opportunity for cost-effective trial execution with AI support, despite facing challenges in data standardization and infrastructure development in some areas.
- Latin America: This region is characterized by nascent but growing adoption, driven by the increasing need to optimize clinical trials and improve healthcare outcomes. Brazil and Mexico are leading the charge with efforts to modernize their healthcare systems and attract foreign investment in pharmaceutical R&D. Challenges include regulatory complexities and limited access to advanced technological infrastructure.
- Middle East & Africa (MEA): The MEA region is experiencing gradual growth, with initiatives in countries like the UAE and Saudi Arabia to diversify their economies and invest heavily in healthcare and technological innovation. While still in early stages, the potential for AI in optimizing clinical research is recognized, particularly in managing chronic diseases and leveraging digital transformation strategies.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the AI in Clinical Trials Market.- IBM Watson Health
- Google Cloud Life Sciences
- NVIDIA Corporation
- IQVIA
- Medidata Solutions (Dassault Systèmes)
- Insilico Medicine
- BenevolentAI
- Owkin
- Exscientia
- ConcertAI
- Deep Genomics
- SAS Institute
- Tempus Labs
- BioXcel Therapeutics
- Antidote Technologies
Frequently Asked Questions
Analyze common user questions about the AI in Clinical Trials market and generate a concise list of summarized FAQs reflecting key topics and concerns.How does AI accelerate drug development timelines?
AI accelerates drug development by automating data analysis, optimizing trial design, streamlining patient recruitment, and predicting outcomes, significantly reducing the time taken for each phase of clinical trials.
What are the primary benefits of integrating AI into clinical trials?
The primary benefits include reduced costs, faster trial completion, higher success rates for drug candidates, improved data accuracy, enhanced patient safety, and the ability to develop more personalized treatments.
Are there significant ethical concerns regarding AIs role in clinical research?
Yes, ethical concerns primarily revolve around data privacy and security, algorithmic bias in patient selection, the transparency and explainability of AI decisions, and the need for robust human oversight to ensure patient well-being and equitable treatment.
Which therapeutic areas are most impacted by AI in clinical trials?
Oncology, neurological disorders, rare diseases, and infectious diseases are among the most impacted. AIs ability to handle complex data and identify subtle patterns is particularly beneficial for these challenging therapeutic areas, accelerating personalized medicine.
What are the main challenges hindering the widespread adoption of AI in clinical trials?
Key challenges include stringent regulatory requirements, difficulties in data integration and standardization, a shortage of skilled AI and clinical research professionals, high initial implementation costs, and the need for validation of AI algorithms in diverse clinical settings.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager