and PEGylated Drugs Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429894 | Date : Nov, 2025 | Pages : 258 | Region : Global | Publisher : MRU
and PEGylated Drugs Market Size
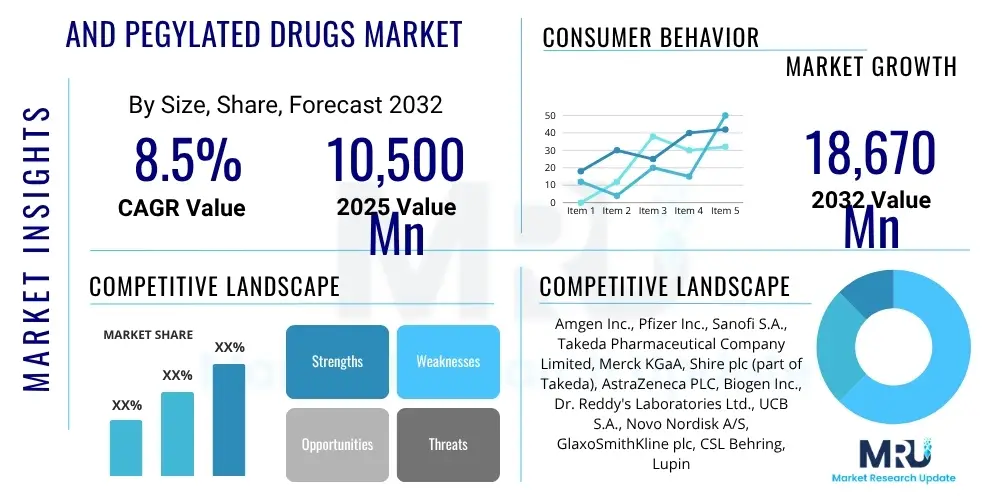
The PEGylated Drugs Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.5% between 2025 and 2032. The market is estimated at USD 10,500 million in 2025 and is projected to reach USD 18,670 million by the end of the forecast period in 2032.
PEGylated Drugs Market introduction
The PEGylated Drugs Market encompasses a specialized segment within pharmaceuticals, leveraging polyethylene glycol (PEG) conjugation to enhance therapeutic properties of various active pharmaceutical ingredients (APIs). PEGylation involves covalently attaching one or more PEG chains to drug molecules, including proteins, peptides, and small molecules, which fundamentally alters their pharmacokinetic and pharmacodynamic profiles. This modification primarily extends the systemic circulation half-life of drugs by reducing renal clearance and protecting them from enzymatic degradation, thereby improving efficacy, reducing dosing frequency, and enhancing patient compliance. Major applications span critical therapeutic areas such as oncology, diabetes, autoimmune diseases, infectious diseases, and growth hormone deficiency, addressing significant unmet medical needs. The substantial benefits derived from PEGylation, including improved stability, reduced immunogenicity, and enhanced solubility, are significant driving factors for market growth. This innovation allows for the development of superior versions of existing drugs and enables the progression of novel therapeutics that might otherwise be unviable due to rapid clearance or poor bioavailability.
PEGylated Drugs Market Executive Summary
The PEGylated Drugs Market is experiencing robust expansion driven by increasing research and development activities in biologics, the rising global prevalence of chronic diseases, and technological advancements in drug delivery systems. Business trends indicate a strong focus on strategic collaborations, mergers, and acquisitions among pharmaceutical companies to expand product portfolios and leverage specialized PEGylation technologies. Regional trends highlight North America and Europe as dominant forces due to established pharmaceutical infrastructure, high healthcare expenditure, and significant R&D investments, while the Asia Pacific region is emerging as a high-growth market propelled by improving healthcare access, growing patient populations, and increasing government support for pharmaceutical innovation. Segment trends show a consistent demand for PEGylated drugs in oncology and diabetes, with an escalating interest in exploring PEGylation for gene therapies, cell therapies, and specialized biosimilars, signaling a dynamic and evolving landscape with diverse opportunities across various therapeutic modalities and geographic regions.
AI Impact Analysis on PEGylated Drugs Market
User inquiries concerning AI's influence on the PEGylated Drugs Market frequently revolve around its potential to revolutionize drug discovery and development, optimize the PEGylation process itself, and enhance precision medicine applications. Key themes identified include the acceleration of R&D timelines, the ability to predict optimal PEGylation sites and polymer characteristics, and the personalization of treatment regimens based on individual patient responses. Concerns often touch upon data security, the ethical implications of AI-driven drug design, and the need for robust validation protocols for AI-generated insights. Expectations are high for AI to reduce development costs, improve success rates in clinical trials, and ultimately lead to more effective and safer PEGylated therapeutics. This transformative technology is anticipated to streamline complex molecular design, improve manufacturing efficiencies, and offer a deeper understanding of drug-target interactions, fostering a new era of innovation in biopharmaceutical development.
- AI-driven optimization of PEGylation parameters: Predicting optimal PEG chain length, branching, and attachment sites to maximize therapeutic efficacy and minimize side effects.
- Accelerated drug discovery and design: AI algorithms can rapidly screen potential drug candidates and predict their suitability for PEGylation, reducing preclinical development time.
- Enhanced clinical trial design and patient selection: AI can analyze vast patient data to identify biomarkers for improved patient stratification, leading to more targeted and efficient clinical trials for PEGylated drugs.
- Improved manufacturing processes: AI can optimize bioprocesses for PEGylation, ensuring higher yields, purity, and consistency of the final drug product, reducing production costs.
- Prediction of drug-drug interactions and adverse effects: AI models can forecast potential interactions and side effects of PEGylated drugs, improving patient safety profiles.
- Personalized medicine approaches: AI facilitates the development of PEGylated drugs tailored to individual patient genetic profiles and disease characteristics, enhancing therapeutic outcomes.
- Regulatory intelligence and compliance: AI tools assist in navigating complex regulatory landscapes, ensuring that PEGylated drug development adheres to global guidelines and accelerates market access.
- Data analysis for pharmacokinetics and pharmacodynamics: AI can process large datasets from in vitro and in vivo studies to better understand drug absorption, distribution, metabolism, and excretion (ADME) of PEGylated compounds.
DRO & Impact Forces Of PEGylated Drugs Market
The PEGylated Drugs Market is significantly influenced by a dynamic interplay of driving forces, inherent restraints, and emerging opportunities that collectively shape its trajectory and impact the pharmaceutical landscape. The escalating global prevalence of chronic diseases such as cancer, diabetes, and autoimmune disorders is a primary driver, fueling demand for innovative and effective long-acting therapeutics. Advances in biotechnology and pharmaceutical R&D, coupled with increasing investments in biologics and biosimilars, further propel market growth by expanding the pipeline of PEGylation candidates. However, the market faces notable restraints, including the high cost and complexity associated with the research, development, and manufacturing of PEGylated drugs. Regulatory challenges, potential immunogenicity issues, and the rigorous requirements for demonstrating improved safety and efficacy over existing therapies also pose significant hurdles. Despite these challenges, substantial opportunities exist in the development of novel PEGylated drug delivery systems, the expansion into orphan drug indications, and the penetration of lucrative emerging markets. The continuous demand for therapies with enhanced pharmacokinetic profiles, reduced dosing frequency, and improved patient compliance underscores the impactful forces driving innovation and commercial success in this specialized pharmaceutical sector.
Segmentation Analysis
The PEGylated Drugs Market is broadly segmented based on drug type, application, and end-user, providing a granular view of market dynamics and growth potential across various categories. Each segment reflects distinct characteristics, driving factors, and competitive landscapes, catering to specific therapeutic needs and patient populations. Understanding these segmentations is critical for market players to develop targeted strategies, optimize product development, and capture specific market shares effectively. The diverse applications of PEGylation allow for a wide range of drug types to benefit from this technology, extending its reach across numerous disease indications and healthcare settings.
- By Drug Type
- Proteins
- Granulocyte Colony-Stimulating Factor (G-CSF)
- Interferons
- Erythropoietins
- Enzymes
- Antibodies
- Peptides
- Glucagon-Like Peptide-1 (GLP-1) Analogs
- Other Therapeutic Peptides
- Small Molecules
- Chemotherapeutics
- Anti-inflammatory Agents
- Other Small Molecule Drugs
- Nucleic Acids and Gene Therapies
- Proteins
- By Application
- Oncology
- Leukemia
- Solid Tumors
- Other Cancers
- Diabetes
- Autoimmune Diseases
- Rheumatoid Arthritis
- Crohn's Disease
- Psoriasis
- Infectious Diseases
- Hepatitis
- HIV
- Other Viral/Bacterial Infections
- Gastrointestinal Disorders
- Growth Hormone Deficiency
- Blood Disorders
- Other Applications
- Oncology
- By End-User
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
- Research and Academic Institutions
- Contract Research Organizations (CROs)
- By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Value Chain Analysis For PEGylated Drugs Market
The value chain for the PEGylated Drugs Market encompasses a complex series of interconnected stages, beginning from the initial discovery and synthesis of active pharmaceutical ingredients (APIs) and polyethylene glycol (PEG) reagents, through to the final distribution and patient consumption. Upstream activities involve extensive research and development for novel drug candidates, advanced PEGylation technologies, and the high-quality manufacturing of specialized PEG polymers by chemical suppliers. Midstream processes include the intricate conjugation of PEG to drug molecules, rigorous analytical characterization to ensure product integrity, formulation development, and extensive preclinical and clinical testing to demonstrate safety and efficacy. Downstream activities are crucial for market access and include obtaining regulatory approvals from health authorities worldwide, large-scale commercial manufacturing, comprehensive marketing and sales strategies, and the establishment of efficient distribution channels. The distribution network typically involves a mix of direct sales to large hospital systems and indirect channels through pharmaceutical wholesalers, distributors, and retail pharmacies, ensuring the broad availability of these specialized therapeutics to end-users globally. Each stage adds significant value, requiring specialized expertise, substantial investment, and adherence to stringent quality and regulatory standards.
PEGylated Drugs Market Potential Customers
Potential customers for PEGylated drugs primarily comprise entities within the healthcare ecosystem and research community seeking advanced therapeutic solutions for chronic and complex diseases. These end-users are driven by the need for drugs with enhanced efficacy, improved safety profiles, and convenient dosing regimens. Hospitals, particularly those with specialized departments such as oncology, endocrinology, and rheumatology, represent a significant customer base due to their direct patient care responsibilities and high volume of prescriptions for chronic conditions. Specialty clinics focusing on specific disease areas, such as diabetes management centers or immunology clinics, also form a crucial segment of buyers. Additionally, government healthcare programs and private insurance providers, which influence drug adoption and reimbursement policies, indirectly serve as key stakeholders. Research and academic institutions, along with pharmaceutical and biotechnology companies involved in drug development, are also potential customers for raw PEGylated components or contract manufacturing services, contributing to the ongoing innovation and expansion of the market.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 10,500 million |
| Market Forecast in 2032 | USD 18,670 million |
| Growth Rate | 8.5% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Amgen Inc., Pfizer Inc., Sanofi S.A., Takeda Pharmaceutical Company Limited, Merck KGaA, Shire plc (part of Takeda), AstraZeneca PLC, Biogen Inc., Dr. Reddy's Laboratories Ltd., UCB S.A., Novo Nordisk A/S, GlaxoSmithKline plc, CSL Behring, Lupin Limited, Sun Pharmaceutical Industries Ltd., Alnylam Pharmaceuticals Inc., Horizon Therapeutics Public Limited Company, Sandoz International GmbH, Fujifilm Diosynth Biotechnologies, Ipsen Pharma. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
PEGylated Drugs Market Key Technology Landscape
The technological landscape of the PEGylated Drugs Market is characterized by continuous innovation aimed at optimizing PEGylation chemistry, enhancing drug delivery, and improving analytical characterization. Advancements in PEGylation techniques involve developing site-specific conjugation methods that ensure precise attachment of PEG chains, minimizing heterogeneity and preserving drug activity. This includes enzymatic PEGylation, click chemistry, and self-assembling PEG conjugates, which offer greater control over the final product's molecular structure and pharmacokinetic properties. Furthermore, the evolution of novel PEG polymers, such as branched, multi-armed, or releasable PEGs, allows for tailored drug release profiles and targeted delivery, expanding the therapeutic potential. The integration of sophisticated analytical technologies, including advanced chromatography, mass spectrometry, and nuclear magnetic resonance (NMR) spectroscopy, is crucial for thorough characterization of PEGylated products, ensuring their purity, stability, and biological activity. Moreover, the increasing adoption of high-throughput screening and computational modeling, particularly with AI and machine learning, is accelerating the identification of optimal PEGylation strategies and drug candidates, significantly impacting the efficiency and success rates in the development pipeline. These technological strides collectively enhance the safety, efficacy, and manufacturability of PEGylated drugs, driving market growth and addressing complex medical needs.
Regional Highlights
- North America: This region holds a dominant share in the PEGylated Drugs Market, primarily due to the presence of major pharmaceutical companies, extensive R&D investments, and a robust healthcare infrastructure. High prevalence of chronic diseases, favorable reimbursement policies, and early adoption of advanced therapeutics further contribute to its leading position. The United States, in particular, is a significant contributor to market revenue, driven by innovative drug development and a strong regulatory framework.
- Europe: Europe represents another substantial market for PEGylated drugs, characterized by a well-established pharmaceutical industry, significant public and private healthcare spending, and a growing aging population susceptible to chronic ailments. Countries like Germany, France, and the UK are at the forefront of pharmaceutical research and development, fostering the introduction and adoption of PEGylated therapies. Regulatory bodies like the EMA play a crucial role in ensuring market access for these advanced treatments.
- Asia Pacific (APAC): The APAC region is projected to witness the highest growth rate in the PEGylated Drugs Market, propelled by increasing healthcare expenditure, improving healthcare access, and a vast patient pool. Emerging economies such as China and India are rapidly expanding their pharmaceutical manufacturing capabilities and R&D activities. Growing awareness about advanced therapeutics and increasing investments in biopharmaceutical infrastructure are key drivers in this region, alongside a rise in chronic disease prevalence.
- Latin America: This region is an emerging market for PEGylated drugs, with countries like Brazil and Mexico showing considerable potential. Market growth is attributed to improving economic conditions, expanding healthcare services, and a rising demand for specialty drugs. However, market penetration is often challenged by affordability concerns and varied regulatory landscapes, necessitating localized strategies for pharmaceutical companies.
- Middle East and Africa (MEA): The MEA region is experiencing gradual growth in the PEGylated Drugs Market, primarily driven by increasing investments in healthcare infrastructure and rising awareness about modern medical treatments, particularly in the Gulf Cooperation Council (GCC) countries. The market is influenced by the adoption of advanced therapies for chronic diseases, although access and affordability remain key considerations. Strategic partnerships and local manufacturing initiatives are pivotal for market expansion here.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the PEGylated Drugs Market.- Amgen Inc.
- Pfizer Inc.
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- Merck KGaA
- Shire plc (part of Takeda)
- AstraZeneca PLC
- Biogen Inc.
- Dr. Reddy's Laboratories Ltd.
- UCB S.A.
- Novo Nordisk A/S
- GlaxoSmithKline plc
- CSL Behring
- Lupin Limited
- Sun Pharmaceutical Industries Ltd.
- Alnylam Pharmaceuticals Inc.
- Horizon Therapeutics Public Limited Company
- Sandoz International GmbH
- Fujifilm Diosynth Biotechnologies
- Ipsen Pharma
Frequently Asked Questions
What are PEGylated drugs?
PEGylated drugs are pharmaceutical compounds that have been covalently attached to one or more chains of polyethylene glycol (PEG), a non-toxic, non-immunogenic, and water-soluble polymer. This modification alters the drug's pharmacokinetic and pharmacodynamic properties, primarily by increasing its molecular size, which reduces renal clearance and protects it from enzymatic degradation, thereby extending its circulation half-life and improving therapeutic efficacy.
What are the primary benefits of PEGylation?
The primary benefits of PEGylation include a significant extension of the drug's half-life in the bloodstream, leading to reduced dosing frequency and enhanced patient compliance. It also reduces immunogenicity and antigenicity of protein and peptide drugs, decreases toxicity, improves drug solubility, and enhances stability against enzymatic degradation, ultimately resulting in a more effective and safer therapeutic profile.
In which therapeutic areas are PEGylated drugs most commonly used?
PEGylated drugs are most commonly utilized across a broad spectrum of therapeutic areas, including oncology for various cancers (e.g., leukemia, solid tumors), diabetes for long-acting insulin and GLP-1 analogs, autoimmune diseases like rheumatoid arthritis and Crohn's disease, and infectious diseases such as hepatitis. They are also vital in treating growth hormone deficiency and certain hematological disorders, leveraging their extended action and improved patient tolerance.
What is the future outlook for the PEGylated drugs market?
The future outlook for the PEGylated drugs market is highly promising, driven by ongoing advancements in biotechnology, increasing R&D investments in biologics, and the rising prevalence of chronic diseases globally. Innovations in site-specific PEGylation, the development of novel PEG polymers, and the application of AI in drug design are expected to further expand the market. Furthermore, the growing demand for biosimilars and the exploration of PEGylation in gene and cell therapies will create new avenues for growth and therapeutic applications.
How does PEGylation improve drug safety and patient compliance?
PEGylation enhances drug safety by reducing immunogenicity, meaning the body is less likely to recognize the drug as foreign and mount an immune response, which minimizes adverse reactions. It also lowers toxicity by improving drug distribution and reducing peak concentrations. For patient compliance, the extended half-life of PEGylated drugs allows for less frequent dosing (e.g., weekly or bi-weekly instead of daily), significantly simplifying treatment regimens and improving adherence to prescribed therapies, especially for chronic conditions.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
