Asthma Spacers Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 428062 | Date : Oct, 2025 | Pages : 242 | Region : Global | Publisher : MRU
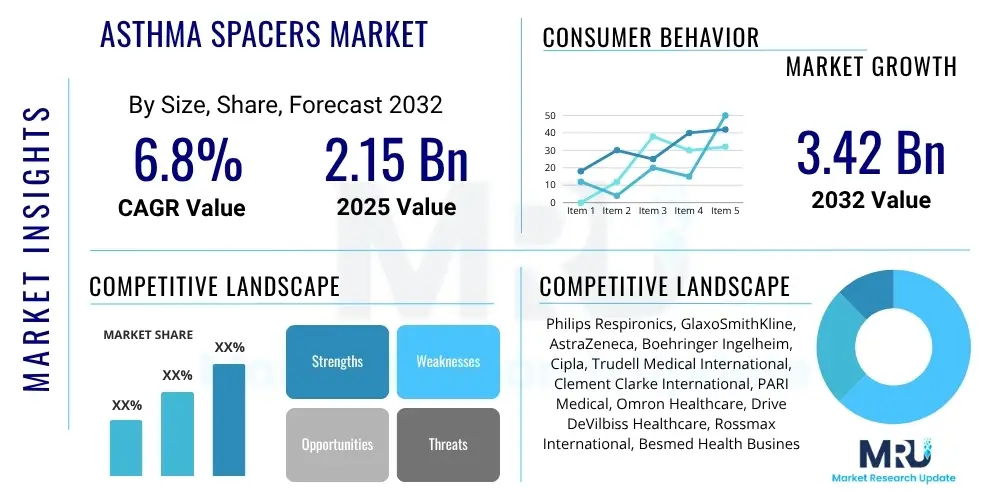
Asthma Spacers Market Size
The Asthma Spacers Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2025 and 2032. The market is estimated at USD 2.15 Billion in 2025 and is projected to reach USD 3.42 Billion by the end of the forecast period in 2032.
Asthma Spacers Market introduction
The Asthma Spacers Market encompasses devices designed to improve the delivery of aerosolized medication from metered-dose inhalers (MDIs) to the lungs. These devices consist of a holding chamber that temporarily stores the medication, allowing the patient to inhale it slowly and deeply, thereby enhancing drug deposition in the airways and minimizing throat deposition. Asthma spacers are crucial tools in the management of chronic respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD), particularly for individuals who struggle with coordinating MDI actuation and inhalation. Their application spans various patient demographics, from pediatric patients who benefit from integrated masks to adults seeking more efficient and consistent medication delivery.
The primary benefit of using an asthma spacer is the significant improvement in the therapeutic efficacy of inhaled medications. By separating the MDI actuation from the inhalation maneuver, spacers reduce the "cold freon" effect and ensure that a greater proportion of the active drug reaches the lower airways, where it is most needed. This not only optimizes treatment outcomes but also helps to minimize systemic side effects associated with poor inhalation technique, such as oral thrush from corticosteroids. The market is propelled by several key driving factors, including the escalating global prevalence of asthma and COPD, increased patient and healthcare provider awareness regarding proper inhaler technique, and continuous advancements in product design and materials that enhance user-friendliness and efficacy. Furthermore, an aging global population, which often faces challenges with MDI coordination, contributes significantly to the demand for these assistive devices.
Asthma Spacers Market Executive Summary
The Asthma Spacers Market is experiencing robust growth, driven by an increasing incidence of respiratory diseases and a heightened focus on effective medication delivery. Current business trends indicate a move towards more patient-centric designs, including anti-static materials, flow-vu indicators, and integration with digital health platforms to improve adherence and treatment outcomes. Consolidations and strategic partnerships among key manufacturers are also shaping the competitive landscape, aiming to broaden product portfolios and expand geographical reach. Innovation in manufacturing processes to reduce costs and enhance product durability remains a critical focus for market players, ensuring wider accessibility of these essential medical devices.
Regionally, developed markets such as North America and Europe continue to dominate due to well-established healthcare infrastructures, high awareness levels, and favorable reimbursement policies. However, the Asia Pacific region is rapidly emerging as a significant growth hub, propelled by its vast patient population, improving healthcare access, and increasing healthcare expenditure. Within market segments, the pediatric category remains a substantial driver, as spacers are particularly beneficial for children who often find it challenging to use MDIs correctly. Furthermore, there is a growing trend towards homecare settings, increasing the demand for portable and easy-to-use devices, while digital integration with smart spacers represents a nascent but rapidly expanding segment, promising personalized patient management and data-driven insights.
AI Impact Analysis on Asthma Spacers Market
The integration of Artificial Intelligence (AI) into the Asthma Spacers Market holds transformative potential, addressing key challenges related to patient adherence, personalized therapy, and remote monitoring. Users are increasingly seeking solutions that move beyond passive drug delivery, desiring devices that offer feedback, track usage patterns, and provide actionable insights for better disease management. Common questions revolve around how AI can enhance the user experience, predict asthma exacerbations, and optimize the overall treatment regimen. There is significant interest in understanding how smart spacers, powered by AI algorithms, can translate raw usage data into meaningful information for both patients and healthcare providers, fostering a proactive approach to respiratory care.
AI's influence is expected to streamline medication adherence through smart device connectivity, which can log inhalation events, provide reminders, and analyze technique. This data can then be transmitted to healthcare professionals, enabling personalized adjustments to treatment plans based on real-world usage patterns. Furthermore, AI-driven predictive analytics could leverage usage data, environmental factors, and patient health records to anticipate potential asthma attacks, allowing for timely interventions. This proactive paradigm shift from reactive treatment to preventive care represents a major expectation from AI integration. By providing intelligent feedback and continuous monitoring, AI will empower patients to take a more active role in managing their condition, ultimately improving health outcomes and reducing the burden of respiratory illnesses.
- AI can enable personalized feedback on inhalation technique, optimizing drug delivery efficiency.
- Predictive analytics, driven by AI, can identify patterns leading to asthma exacerbations, enabling proactive intervention.
- Smart spacers with AI integration can track medication adherence, offering real-time data for patients and healthcare providers.
- AI can integrate spacer usage data with broader health ecosystems, including electronic health records and telemedicine platforms.
- Development of AI-powered educational tools within spacer apps to improve patient understanding and self-management.
- Facilitation of remote monitoring and virtual consultations through AI-enabled data sharing from smart spacers.
DRO & Impact Forces Of Asthma Spacers Market
The Asthma Spacers Market is significantly influenced by a dynamic interplay of drivers, restraints, opportunities, and various impact forces. The primary drivers fueling market expansion include the consistently rising global prevalence of chronic respiratory diseases like asthma and COPD, necessitating effective drug delivery solutions. Enhanced awareness among patients and healthcare professionals about the benefits of spacers in improving medication efficacy and reducing side effects also plays a crucial role. Furthermore, continuous product innovations, such as the development of anti-static materials and user-friendly designs, coupled with supportive government initiatives and favorable reimbursement policies in developed economies, are pivotal in sustaining market growth. The increasing adoption of telemedicine and home healthcare further expands the reach and utility of these devices.
Conversely, several restraints impede the market's full potential. The relatively high cost of advanced or "smart" spacers, especially in price-sensitive emerging markets, can limit adoption. A persistent lack of awareness regarding proper inhaler technique and the importance of spacers in certain regions, particularly developing countries, continues to be a hurdle. The availability of alternative drug delivery methods, such as nebulizers and dry powder inhalers (DPIs), also presents competition. Moreover, issues related to patient compliance in routine cleaning and maintenance of spacers can impact their long-term effectiveness and patient satisfaction. Overcoming these challenges requires concerted efforts in patient education, affordability strategies, and technological advancements.
Opportunities for growth are abundant, particularly in emerging markets with large underserved populations and rapidly improving healthcare infrastructures. The integration of smart technology into spacers, offering features like Bluetooth connectivity, usage tracking, and AI-driven feedback, presents a substantial opportunity for product differentiation and enhanced patient outcomes. Strategic partnerships with pharmaceutical companies to bundle spacers with MDIs, as well as expansion into new therapeutic areas beyond asthma and COPD, represent avenues for market penetration. From an impact forces perspective, the bargaining power of buyers (large hospital groups, national healthcare systems) can influence pricing, while the bargaining power of suppliers (raw material providers) affects manufacturing costs. The threat of new entrants, while moderate due to regulatory hurdles and established brand loyalty, encourages continuous innovation. The competitive rivalry among existing players is intense, driving product development and market consolidation, while the threat of substitutes, particularly with the evolution of DPIs and nebulizer technology, keeps pressure on spacer manufacturers to demonstrate superior efficacy and user convenience.
Segmentation Analysis
The Asthma Spacers Market is comprehensively segmented to address the diverse needs of its user base and delineate various market dynamics. These segments allow for a detailed understanding of consumer preferences, technological advancements, and regional variations in adoption patterns. Key differentiators include the type of device, the intended end-user, the channels through which products are distributed, the materials used in their construction, and the specific age group they cater to. Each segmentation provides unique insights into market drivers and competitive strategies, enabling targeted product development and marketing initiatives across the global landscape.
- By Product Type
- Valved Holding Chambers (VHCs)
- Non-Valved Spacers
- By End-User
- Hospitals & Clinics
- Homecare Settings
- Emergency Medical Services (EMS)
- By Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
- Online Pharmacies
- By Material
- Plastic (Polycarbonate, Polypropylene)
- Metal
- Silicone
- By Patient Age Group
- Pediatric
- Adult
Value Chain Analysis For Asthma Spacers Market
The value chain for the Asthma Spacers Market is a complex network of activities that transforms raw materials into finished products and delivers them to the end-users. It begins with upstream analysis, involving the sourcing of raw materials such as medical-grade plastics (polycarbonate, polypropylene), silicone, and sometimes metal components for valves or specialized parts. Key suppliers in this stage are chemical companies and manufacturers of precision components. Research and Development (R&D) is a critical component, focusing on improving material properties, optimizing design for better drug delivery, enhancing user comfort, and integrating smart technologies. Manufacturing processes involve injection molding, assembly, quality control, and sterilization, requiring specialized facilities and adherence to stringent medical device regulations.
Further along the value chain, downstream analysis highlights the distribution and sales channels. Manufacturers often rely on a network of wholesalers, distributors, and pharmaceutical representatives to reach various market segments. These intermediaries play a crucial role in inventory management, logistics, and market penetration, especially in diverse geographical regions. The distribution channel can be direct, where manufacturers sell directly to large hospital networks, government healthcare programs, or key opinion leaders for bulk procurement. This direct approach allows for better control over pricing and customer relationships, often involving dedicated sales forces and extensive customer support.
Indirect distribution, on the other hand, involves leveraging a wider network of retail pharmacies, hospital pharmacies, and increasingly, online pharmacies. Retail pharmacies offer widespread accessibility to individual patients, while hospital pharmacies serve institutional needs. Online pharmacies are gaining significant traction, providing convenience, competitive pricing, and a broader reach, particularly for recurring purchases. These indirect channels require robust partnerships, effective supply chain management, and often involve marketing and educational initiatives aimed at both pharmacists and consumers to drive product adoption and ensure proper usage. Effective collaboration across all stages of this value chain is paramount for ensuring product quality, market reach, and ultimately, patient satisfaction.
Asthma Spacers Market Potential Customers
The Asthma Spacers Market serves a diverse range of potential customers, primarily focusing on individuals afflicted with respiratory conditions that necessitate the use of metered-dose inhalers. The foremost end-users are patients diagnosed with asthma and chronic obstructive pulmonary disease (COPD), spanning all age groups from infants and young children to adults and the elderly. Pediatric patients represent a particularly significant segment, as spacers, especially those equipped with masks, are indispensable for ensuring effective medication delivery due to their difficulty in coordinating inhalation and MDI actuation. Adult patients who face challenges with proper MDI technique, or those requiring high doses of inhaled corticosteroids, also form a substantial customer base, benefiting from improved drug deposition and reduced systemic side effects.
Beyond individual patients, healthcare providers constitute another vital segment of potential customers. This includes hospitals, clinics, general practitioners, pulmonologists, and emergency medical services, all of whom prescribe, recommend, or directly administer asthma spacers as part of their standard treatment protocols. These institutional buyers are often concerned with device efficacy, durability, ease of cleaning, and cost-effectiveness for bulk procurement. Furthermore, pharmacists play a crucial role as key influencers and distributors, often educating patients on proper spacer usage and recommending specific products. Caregivers, parents of pediatric patients, and guardians of elderly individuals also represent an indirect but important customer group, as their purchasing decisions are often driven by recommendations from healthcare professionals and the desire to ensure optimal treatment outcomes for their dependents.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 2.15 Billion |
| Market Forecast in 2032 | USD 3.42 Billion |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Philips Respironics, GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Cipla, Trudell Medical International, Clement Clarke International, PARI Medical, Omron Healthcare, Drive DeVilbiss Healthcare, Rossmax International, Besmed Health Business, Sanofi, Teva Pharmaceutical Industries, Viatris Inc. (Mylan), Sunovion Pharmaceuticals Inc., Beximco Pharmaceuticals Ltd., Grifols S.A., 3M Company, Baxter International Inc. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Asthma Spacers Market Key Technology Landscape
The technological landscape of the Asthma Spacers Market is characterized by continuous innovation aimed at enhancing drug delivery efficacy, improving patient adherence, and increasing user convenience. A fundamental advancement has been the development of valved holding chambers (VHCs), which incorporate one-way valves to retain the aerosolized medication, allowing patients to inhale at their own pace without concerns about timing the MDI actuation. Material science plays a significant role, with the introduction of anti-static plastics that prevent medication from sticking to the chamber walls, thereby ensuring a consistent and higher dose delivery to the lungs. Furthermore, design innovations include integrated masks for pediatric patients, ergonomic shapes for comfortable handling, and visual indicators (like flow-vu indicators) that provide feedback on correct inhalation technique, empowering patients to self-monitor their usage.
The emerging frontier in asthma spacer technology involves the integration of digital and smart features. This includes Bluetooth-enabled spacers that can connect to smartphone applications, allowing for the tracking of medication usage, reminders for daily doses, and monitoring of inhalation technique. These smart devices can collect data on adherence patterns, providing valuable insights for both patients and healthcare providers to tailor treatment plans. Sensor technology embedded within spacers can detect inhalation flow rates and volumes, offering real-time feedback and improving patient education on optimal breathing maneuvers. The ability of these smart spacers to interface with telehealth platforms and electronic health records further solidifies their role in a connected healthcare ecosystem, facilitating remote patient monitoring and improving chronic disease management through data-driven approaches. These technological advancements not only improve the clinical outcomes for patients but also differentiate products in a competitive market, setting new standards for respiratory care.
Regional Highlights
- North America: This region holds a significant share of the Asthma Spacers Market, driven by a high prevalence of asthma and COPD, robust healthcare infrastructure, and high awareness regarding advanced respiratory care. Favorable reimbursement policies, significant R&D investments, and the presence of key market players contribute to its dominance. The United States and Canada are leading countries, characterized by early adoption of new technologies and a strong focus on patient education for effective chronic disease management.
- Europe: Europe is another prominent market for asthma spacers, characterized by established healthcare systems, increasing geriatric population susceptible to respiratory conditions, and stringent regulatory frameworks ensuring product quality. Countries like Germany, the UK, France, and Italy exhibit high market penetration, supported by public healthcare funding and extensive awareness campaigns promoting proper inhaler use. Innovations in smart spacer technology and sustainable materials are gaining traction.
- Asia Pacific (APAC): The APAC region is projected to be the fastest-growing market due to its vast population, rising incidence of respiratory diseases, improving healthcare expenditure, and increasing access to advanced medical devices. Countries such as China, India, Japan, and Australia are key contributors to this growth. Economic development and rising disposable incomes are leading to better healthcare access and greater adoption of sophisticated medical solutions, despite challenges related to awareness and affordability in some rural areas.
- Latin America: The market in Latin America is experiencing gradual growth, influenced by improving healthcare facilities and increasing awareness of respiratory diseases. Brazil, Mexico, and Argentina are leading the regional market. Economic volatility and varying healthcare access remain challenges, but government initiatives to improve public health and increase access to essential medicines are creating new opportunities for market expansion.
- Middle East and Africa (MEA): The MEA region is an emerging market for asthma spacers, driven by improving healthcare infrastructure, rising prevalence of asthma, and increasing investments in healthcare. The GCC countries (Saudi Arabia, UAE) are leading in adopting advanced medical technologies, while parts of Africa are experiencing growth due to international aid and efforts to strengthen public health systems. Challenges include limited awareness, affordability, and the need for greater penetration of modern healthcare practices.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Asthma Spacers Market.- Philips Respironics
- GlaxoSmithKline
- AstraZeneca
- Boehringer Ingelheim
- Cipla
- Trudell Medical International
- Clement Clarke International
- PARI Medical
- Omron Healthcare
- Drive DeVilbiss Healthcare
- Rossmax International
- Besmed Health Business
- Sanofi
- Teva Pharmaceutical Industries
- Viatris Inc. (Mylan)
- Sunovion Pharmaceuticals Inc.
- Beximco Pharmaceuticals Ltd.
- Grifols S.A.
- 3M Company
- Baxter International Inc.
Frequently Asked Questions
Analyze common user questions about the Asthma Spacers market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is an asthma spacer and how does it work to improve medication delivery?
An asthma spacer is a hollow tube or chamber, typically made of plastic, designed to be used with a metered-dose inhaler (MDI). It holds the medication in a suspended cloud for a few seconds after the inhaler is actuated, allowing the patient to inhale the drug slowly and deeply. This mechanism improves the delivery of medication to the lungs by reducing the speed of the aerosolized drug, minimizing throat deposition, and overcoming coordination difficulties between MDI actuation and inhalation, thereby enhancing the therapeutic effect.
What are the primary benefits of using an asthma spacer compared to using an MDI alone?
The primary benefits of using an asthma spacer include significantly improved drug delivery to the lungs, leading to better clinical outcomes and reduced systemic side effects like oral thrush. Spacers simplify the inhalation process, making MDIs easier to use for children, the elderly, and those with poor coordination. They also ensure a more consistent and effective dose reaches the airways, contributing to enhanced patient compliance and better management of chronic respiratory conditions.
Are there different types of asthma spacers available, and how do they differ?
Yes, there are primarily two types: valved holding chambers (VHCs) and non-valved spacers. VHCs incorporate one-way valves that trap the medication inside the chamber until the patient inhales, offering more flexibility in breathing patterns. Non-valved spacers are simpler tubes that connect the MDI to the mouth. VHCs are generally preferred for their superior drug delivery efficiency and ease of use, especially for pediatric patients who may require a mask attachment.
How often should an asthma spacer be cleaned and when should it be replaced?
An asthma spacer should typically be cleaned at least once a week to prevent the buildup of medication residue, which can affect its efficacy. Cleaning usually involves disassembling the spacer (if possible), washing it in warm, soapy water, rinsing it thoroughly without scrubbing, and allowing it to air dry completely. Most manufacturers recommend replacing spacers every 6 to 12 months, or sooner if there are any signs of damage, cracks, or malfunction in the valves, to ensure optimal performance and hygiene.
How are smart asthma spacers enhancing patient management and adherence?
Smart asthma spacers are integrating digital technologies like Bluetooth connectivity and sensors to enhance patient management and adherence. These devices can track medication usage, monitor inhalation technique, and send data to smartphone applications. This allows patients to receive personalized feedback, set reminders, and share adherence data with healthcare providers, enabling more informed treatment adjustments, predictive analytics for exacerbations, and improved overall engagement in their respiratory care.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
