Bioink Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429937 | Date : Nov, 2025 | Pages : 246 | Region : Global | Publisher : MRU
Bioink Market Size
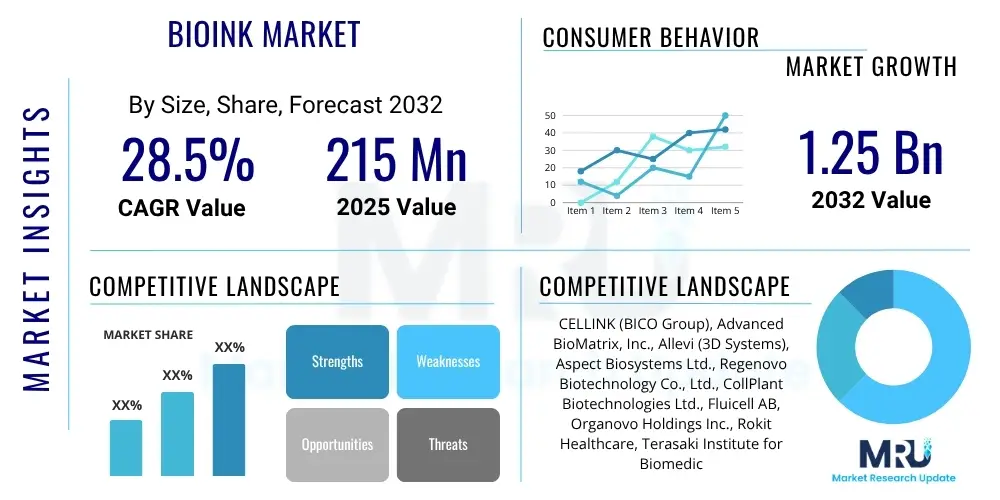
The Bioink Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 28.5% between 2025 and 2032. The market is estimated at $215 Million in 2025 and is projected to reach $1.25 Billion by the end of the forecast period in 2032.
Bioink Market introduction
The Bioink Market represents a pivotal frontier in biomedical engineering, revolutionizing how researchers and clinicians approach tissue repair, regeneration, and drug testing. At its core, bioink refers to a biocompatible material, often a hydrogel, that can be laden with living cells, growth factors, and other biomolecules. These specialized materials are designed to be extruded or jetted with precision by 3D bioprinters, enabling the layer-by-layer fabrication of complex 3D tissue constructs that closely mimic the architecture and function of natural biological tissues. The inherent requirement for bioinks to maintain cell viability, provide structural support, and allow for nutrient and waste exchange makes their formulation a challenging yet rapidly advancing scientific endeavor. The fundamental objective is to create an optimal microenvironment that supports cell proliferation, differentiation, and the eventual maturation of functional tissue structures, propelling the field of regenerative medicine forward.
Product descriptions typically highlight the diverse range of bioink compositions available, tailored for specific cellular environments and printing methods. Common types include natural polymer-based bioinks such as alginate, collagen, gelatin, and hyaluronic acid, which are prized for their excellent biocompatibility, biodegradability, and cellular recognition properties. These natural components often require chemical modification to enhance their mechanical stability and printability. Synthetic polymer bioinks, like PLGA, PEG, and PCL, offer tunable mechanical properties and degradation rates, providing flexibility for various tissue engineering applications where specific mechanical cues are crucial. Hybrid bioinks combine the advantages of both natural and synthetic components, aiming for an optimal balance of biological cues and mechanical robustness. The meticulous selection of a bioink is paramount, directly influencing the success of bioprinting endeavors from basic research to translational applications, and often involves balancing printability with cellular compatibility and long-term tissue function.
The major applications of bioinks span several high-impact areas within healthcare and life sciences, delivering significant benefits. In tissue engineering, bioinks are fundamental for creating scaffolds for bone, cartilage, skin, and vascular repair, offering groundbreaking solutions for organ shortages and chronic disease management by fabricating functional tissue replacements. Drug discovery and toxicology testing benefit immensely from 3D bioprinted tissue models, which provide more physiologically relevant platforms than traditional 2D cell cultures, potentially reducing reliance on animal testing and accelerating drug development timelines by accurately predicting drug efficacy and toxicity. Furthermore, the advent of personalized medicine heavily leverages bioinks to create patient-specific tissue models for diagnostics, therapeutic screening, and ultimately, customized regenerative treatments, thereby enhancing treatment outcomes and driving significant demand across the healthcare ecosystem. The ability to mimic complex biological environments precisely is a key driving factor, pushing the boundaries of what is possible in biomedical research.
Bioink Market Executive Summary
The Bioink Market is experiencing robust growth, driven by escalating demand for advanced regenerative medicine solutions, organ transplantation alternatives, and more physiologically accurate in vitro models for drug development. Emerging business trends include a pronounced shift towards strategic collaborations between academic institutions, biotechnology firms, and pharmaceutical companies, fostering innovation in novel bioink formulations and bioprinting technologies. There is also a significant increase in venture capital investments targeting startups focused on specialized bioinks and integrated bioprinting solutions, indicating a strong entrepreneurial ecosystem. Furthermore, companies are increasingly focusing on scaling up manufacturing capabilities and obtaining regulatory approvals for clinical applications, signaling a transition from pure research to therapeutic commercialization. Customization and patient-specific solutions are becoming key differentiators, propelling businesses to invest in advanced R&D for personalized bioinks.
From a regional perspective, North America and Europe continue to dominate the bioink market, primarily due to well-established healthcare infrastructures, substantial government funding for biomedical research, and the presence of numerous key market players and academic research hubs. These regions benefit from a high adoption rate of advanced biotechnologies and a robust intellectual property landscape. However, the Asia Pacific region is rapidly emerging as a significant growth engine, fueled by rising healthcare expenditures, increasing awareness of advanced therapies, expanding research activities in countries like China, Japan, and South Korea, and a growing patient population. Latin America and the Middle East & Africa regions are also showing nascent growth, driven by improving healthcare facilities and a growing interest in biotechnological advancements, albeit from a lower base, making them attractive future investment areas for market expansion.
Segment trends within the bioink market reveal dynamic shifts and concentrated growth areas. Hydrogel-based bioinks, particularly those derived from natural polymers like alginate, collagen, and gelatin, continue to hold a dominant share due to their excellent biocompatibility and proven track record in various bioprinting applications. However, synthetic and hybrid bioinks are gaining traction as researchers seek materials with more tunable mechanical properties and superior printability for complex tissue structures. Application-wise, tissue engineering and regenerative medicine remain the largest segments, underpinned by the critical need for repairing or replacing damaged tissues and organs. The drug discovery and toxicology testing segment is witnessing the fastest growth, as bioprinted 3D models offer a more reliable and cost-effective alternative to traditional preclinical testing methods, driving investment in novel bioink formulations optimized for disease modeling and high-throughput screening.
AI Impact Analysis on Bioink Market
Users are keenly interested in how Artificial Intelligence (AI) can fundamentally transform the bioink market, extending beyond mere automation to encompass advanced design, optimization, and predictive capabilities. Key user questions frequently revolve around the potential for AI to accelerate bioink formulation discovery, enhance the precision and reproducibility of bioprinting processes, and improve the predictability of tissue engineering outcomes. Common concerns include the complexity of integrating AI tools into existing laboratory workflows, the need for extensive training data, and the ethical implications surrounding autonomous material development. However, expectations are high for AI to deliver significant breakthroughs, such as faster identification of optimal bioink compositions with desired mechanical and biological properties, more accurate simulation of bioprinting dynamics to prevent failures, and automated analysis of bioprinted construct viability and functionality, ultimately reducing research and development costs and accelerating the path to clinical translation. The ability of AI to sift through vast datasets of material properties and biological interactions is perceived as a critical enabler for the next generation of advanced bioinks.
- Accelerated bioink formulation discovery and optimization by predicting ideal material compositions and additives.
- Enhanced precision and resolution in bioprinting processes through AI-driven control of print parameters and nozzle movements.
- Predictive modeling for tissue maturation, functionality, and long-term viability of bioprinted constructs.
- Automated analysis of bioprinted construct characteristics, including cell viability, morphology, and mechanical properties.
- Personalized bioink development based on patient-specific cellular and genetic data for precision medicine applications.
- Streamlined R&D workflows, reducing experimental trial-and-error and speeding up time-to-market for new bioink products.
- Optimization of complex vascularization strategies within larger bioprinted tissues through intelligent design algorithms.
- Improved quality control and consistency in bioink manufacturing through AI-powered anomaly detection.
DRO & Impact Forces Of Bioink Market
The Bioink Market is significantly driven by a confluence of factors that underscore its increasing importance in the biomedical landscape. A primary driver is the rising global prevalence of chronic diseases, such as cardiovascular disorders, diabetes, and organ failures, which necessitate advanced therapeutic interventions like organ transplantation and regenerative medicine. Given the severe shortage of donor organs, bioinks offer a promising avenue for creating patient-specific tissues and organs, thereby addressing a critical unmet medical need. Furthermore, continuous technological advancements in 3D bioprinting technologies, including improved resolution, speed, and multi-material printing capabilities, directly translate to an expanded demand for sophisticated and specialized bioink formulations. Increasing investments in research and development by both public and private sectors in regenerative medicine and tissue engineering further propel market growth, as more functional and clinically viable bioinks are developed and commercialized.
Despite its substantial growth potential, the Bioink Market faces several notable restraints that could impede its trajectory. One significant challenge is the high cost associated with both bioprinting equipment and premium-quality bioinks, which can be prohibitive for smaller research institutions or nascent companies, limiting broader adoption. The complexity and protracted nature of regulatory approval processes for bioprinted tissues and organs, especially those intended for human implantation, represent another substantial hurdle. Regulatory bodies require extensive safety and efficacy data, which can be time-consuming and expensive to generate. Moreover, inherent technical challenges, such as achieving adequate vascularization within large, thick bioprinted tissues to ensure nutrient supply and waste removal, remain a significant scientific and engineering barrier, preventing the full realization of complex organ printing. These restraints necessitate ongoing innovation and collaborative efforts to overcome.
Nonetheless, the Bioink Market is replete with opportunities for growth and innovation. The most prominent opportunity lies in the emergence of novel bioink materials, including smart or stimuli-responsive bioinks that can change properties in response to physiological cues, offering unprecedented control over tissue development. The burgeoning demand for personalized medicine presents a vast market for custom-designed bioinks tailored to individual patient needs, potentially leading to highly effective and less rejected therapies. Advancements in stem cell research, particularly induced pluripotent stem cells (iPSCs), open new avenues for sourcing patient-specific cells for bioinks, overcoming ethical concerns and immunological rejection. Beyond traditional biomedical applications, there is untapped potential in areas such as cosmetic testing (reducing animal testing) and the burgeoning cultured meat industry, where bioinks could play a crucial role in creating structured meat products, diversifying revenue streams for bioink manufacturers and expanding the market's reach.
The market for bioinks is influenced by several powerful impact forces. Technological innovation remains the paramount force, driving the development of more sophisticated bioprinters, novel bioink formulations, and advanced tissue engineering protocols, continually expanding the market's capabilities. The evolving regulatory landscape, as authorities worldwide strive to establish clear guidelines for bioprinted products, significantly shapes market access and commercialization timelines; a clearer framework could accelerate adoption. The level of research funding from governmental bodies, private foundations, and venture capitalists directly impacts the pace of innovation and product development. Ethical considerations surrounding the creation of human tissues and organs, as well as the use of certain cell types, also exert influence on public perception and regulatory scrutiny. Finally, increasing public awareness and acceptance of advanced bioprinting and regenerative therapies are crucial for fostering market demand and facilitating the integration of these technologies into mainstream healthcare.
Segmentation Analysis
The bioink market is broadly segmented by material type, application, and end-user, reflecting the diverse requirements and multifaceted uses of these advanced biomaterials across the life sciences and healthcare sectors. Understanding these segments is crucial for market stakeholders to identify specific growth areas, tailor their product development strategies, and effectively target their marketing efforts. Each segmentation offers unique insights into the market's dynamics, illustrating how different bioink properties are leveraged for distinct purposes, from foundational research to potential clinical applications. The regional segmentation further highlights the geographical distribution of demand and technological advancement, providing a comprehensive view of the global market landscape.
- By Material Type:
- Natural Polymers: Alginate, Collagen, Gelatin, Fibrin, Hyaluronic Acid, Chitosan, Silk Fibroin, Decellularized Extracellular Matrix (dECM)
- Synthetic Polymers: Poly(Lactic-co-Glycolic Acid) (PLGA), Polyethylene Glycol (PEG), Polycaprolactone (PCL), Polylactic Acid (PLA), Polyvinyl Alcohol (PVA)
- Hybrid Bioinks: Combinations of natural and synthetic polymers
- Ceramic Bioinks: Hydroxyapatite, Tricalcium Phosphate (TCP)
- Living Cell Bioinks: Directly incorporating cells within the printing solution
- By Application:
- Tissue Engineering:
- Bone and Cartilage Engineering
- Skin Engineering
- Vascular Engineering
- Nerve Engineering
- Liver Engineering
- Heart Engineering
- Kidney Engineering
- Other Tissues (e.g., Pancreas, Intestine)
- Drug Discovery and Toxicology Testing
- Regenerative Medicine
- Personalized Medicine
- 3D Cell Culture
- Organ Printing
- By End-User:
- Research and Academic Laboratories
- Pharmaceutical and Biotechnology Companies
- Hospitals and Diagnostic Centers
- Cosmetic Industry
- Food Industry
- By Region:
- North America (U.S., Canada)
- Europe (Germany, U.K., France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, Japan, India, South Korea, Australia, Rest of Asia Pacific)
- Latin America (Brazil, Mexico, Rest of Latin America)
- Middle East & Africa (South Africa, GCC Countries, Rest of MEA)
Value Chain Analysis For Bioink Market
The value chain of the Bioink Market commences with a critical upstream analysis focused on the sourcing and preparation of raw materials. This initial stage involves suppliers providing a diverse array of components, including natural polymers like alginate, collagen, and hyaluronic acid; synthetic polymers such as PLGA and PEG; various cell sources, often stem cells or primary cells; growth factors; and other crucial biomolecules and reagents. Ensuring the high purity, consistency, and biocompatibility of these foundational materials is paramount, as any compromise at this stage can significantly impact the performance and safety of the final bioink and bioprinted construct. Reliable sourcing from certified vendors and stringent quality control protocols are essential to guarantee batch-to-batch reproducibility and regulatory compliance, directly influencing the efficacy and clinical translation potential of bioink products.
Following the procurement of raw materials, the manufacturing and formulation stage constitutes the core of the bioink value chain. Bioink manufacturers process these components, often developing proprietary blends and chemical modifications to create formulations optimized for specific bioprinting techniques, cell types, and desired tissue properties. This involves extensive research and development to balance critical factors such as printability (viscosity, gelation kinetics), cell viability and proliferation within the matrix, and the mechanical integrity and biodegradability of the final bioprinted scaffold. Adherence to Good Manufacturing Practices (GMP) and rigorous quality assurance measures throughout the formulation process are vital to ensure the sterility, stability, and consistent performance of bioinks, preparing them for highly sensitive biomedical applications. Customization capabilities, allowing for tailored bioink properties for niche research or clinical requirements, are also a key differentiator at this stage.
The downstream analysis primarily concerns the distribution and application of manufactured bioinks to end-users. Once formulated and packaged, bioinks are distributed to a diverse customer base, including academic research laboratories, pharmaceutical and biotechnology companies, and increasingly, hospitals and diagnostic centers. This distribution can occur through direct sales channels, where manufacturers engage directly with large-volume clients or those requiring specialized technical support and customized solutions. Alternatively, indirect channels involve leveraging specialized distributors or online e-commerce platforms, which provide broader market reach, particularly to smaller research groups or international customers, by utilizing established logistics networks. Efficient logistics, especially maintaining a cold chain for temperature-sensitive cell-laden bioinks, and robust inventory management are crucial to ensure timely delivery and preserve product integrity until it reaches the final application point.
The distinction between direct and indirect distribution channels plays a significant role in market penetration and customer relationship management. Direct channels enable manufacturers to foster closer relationships with key opinion leaders and large institutional buyers, facilitating direct feedback for product improvement and enabling responsive technical assistance. This approach is often favored for high-value, specialized bioinks that require intricate application knowledge. In contrast, indirect channels, through a network of distributors, allow bioink companies to achieve wider market coverage, particularly in regions where they lack a direct sales presence. Distributors often possess local market expertise, established sales forces, and warehousing capabilities, which can significantly reduce a manufacturer's operational overhead and expand customer access. A balanced approach, combining both direct and indirect strategies, often proves most effective for comprehensive market reach and sustained growth within the global bioink industry, adapting to varying customer needs and geographical considerations.
Bioink Market Potential Customers
The primary customers for bioinks encompass a broad spectrum of research and clinical entities, driven by the escalating demand for advanced biological models and regenerative therapies. Academic and government research institutions represent a significant customer segment, continually pushing the boundaries of fundamental research in tissue engineering, regenerative medicine, and drug discovery. These institutions utilize bioinks to develop novel construct designs, investigate cell-material interactions, and create complex in vitro models for disease pathogenesis studies, making them foundational consumers of diverse bioink formulations and associated bioprinting technologies. Their demand often spans a wide range of bioink types, from basic hydrogels to highly customized, cell-laden options, reflecting the exploratory nature of their research objectives.
Pharmaceutical and biotechnology companies constitute another major and rapidly growing customer segment. These industry players leverage bioinks for high-throughput drug screening, toxicology testing, and the development of patient-specific disease models, which offer more physiologically relevant platforms than traditional 2D cell cultures. By enabling the creation of intricate 3D tissue models, bioinks help pharmaceutical firms to more accurately predict drug efficacy and potential adverse effects earlier in the development pipeline, potentially reducing costly late-stage failures and accelerating time-to-market for novel therapeutics. This segment's demand is often for bioinks that are highly reproducible, scalable, and compatible with automated bioprinting systems, facilitating industrial-scale research and development.
Hospitals and diagnostic centers are emerging as key potential customers, particularly as bioprinted tissues and organs move closer to clinical application. As regenerative medicine advances, there is a growing interest in using bioprinted constructs for surgical repair, advanced wound healing, and ultimately, for organ transplantation. Bioinks, therefore, become crucial for creating patient-specific implants or patches, offering superior integration and reduced immune rejection compared to conventional prosthetics or allografts. Furthermore, the cosmetic industry represents a niche but growing customer base, utilizing bioprinted skin models for in vitro testing of products, thereby reducing reliance on animal testing and addressing ethical concerns. The burgeoning cultured meat industry also shows promise as a future consumer, employing bioinks to create structured meat products, diversifying the end-user landscape for bioink manufacturers.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | $215 Million |
| Market Forecast in 2032 | $1.25 Billion |
| Growth Rate | CAGR 28.5% |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | CELLINK (BICO Group), Advanced BioMatrix, Inc., Allevi (3D Systems), Aspect Biosystems Ltd., Regenovo Biotechnology Co., Ltd., CollPlant Biotechnologies Ltd., Fluicell AB, Organovo Holdings Inc., Rokit Healthcare, Terasaki Institute for Biomedical Innovation (TIBI), EnvisionTEC GmbH, GeSiM mbH, Photocentric Ltd., BRIGHTCELLS, Inc., nScrypt Inc., MedPrin Regenerative Medical Technologies Co., Ltd., Eppendorf AG, Merck KGaA, Thermo Fisher Scientific Inc., Sigma-Aldrich (Merck KGaA) |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Bioink Market Key Technology Landscape
The Bioink Market is fundamentally shaped by continuous advancements in bioprinting techniques, which dictate the specific requirements and capabilities of bioink formulations. Extrusion-based bioprinting remains a widely used method due to its versatility with various bioink viscosities and high cell viability, enabling the printing of large and complex structures. Inkjet bioprinting, on the other hand, offers higher resolution and speed, making it suitable for patterning cells and biomolecules with fine detail, though it typically requires lower viscosity bioinks. Laser-assisted bioprinting provides ultra-high resolution and precision, ideal for creating intricate microstructures and direct cell patterning. Emerging technologies like stereolithography (SLA) and digital light processing (DLP) bioprinting are gaining traction for their ability to rapidly fabricate complex 3D structures using photo-crosslinkable bioinks, expanding the geometric complexity and throughput achievable in bioprinting. The selection of the appropriate bioprinting technology is often driven by the desired resolution, speed, cell viability requirements, and the specific application.
Material science innovation forms another crucial pillar of the bioink technology landscape, with researchers constantly striving to develop novel formulations that better mimic the native extracellular matrix while possessing optimal printability and mechanical properties. This includes the engineering of hydrogels with enhanced biocompatibility, tunable stiffness, and controlled degradation rates to support long-term cell growth and tissue maturation. A significant area of focus is the development of "smart" or stimuli-responsive bioinks that can change their properties in response to physiological cues such as pH, temperature, or enzyme activity, allowing for dynamic control over the cellular microenvironment post-printing. The incorporation of growth factors, nanoparticles, or active biomolecules directly into bioink formulations is also a key technological trend, designed to promote specific cellular behaviors, accelerate tissue regeneration, and improve the functional outcomes of bioprinted constructs. These material innovations are critical for overcoming current limitations and expanding the range of clinically viable bioprinting applications.
Artificial Intelligence (AI) and automation are increasingly playing a transformative role across the bioink market's technological landscape, optimizing processes from bioink development to bioprinted tissue assessment. AI-driven platforms are being utilized to rapidly screen and predict optimal bioink compositions by analyzing vast datasets of material properties, cellular responses, and printing parameters, significantly accelerating the discovery and optimization phases. Machine learning algorithms can fine-tune bioprinting protocols, minimizing errors and improving the reproducibility and precision of complex tissue architectures. Automation in bioprinting systems ensures high-throughput fabrication and consistency, critical for scaling up production and moving towards industrial applications. Furthermore, AI contributes to automated quality control and analysis of bioprinted constructs, providing objective assessment of cell viability, morphology, and mechanical characteristics, thereby streamlining validation processes and enhancing the reliability of research and preclinical models. This integration of AI and automation is pivotal for achieving higher efficiency, reducing costs, and accelerating the translation of bioink technologies from lab to clinic.
Regional Highlights
- North America: This region consistently leads the global Bioink Market, primarily driven by a robust and well-established research and development infrastructure, significant government and private funding directed towards regenerative medicine and tissue engineering initiatives, and the strong presence of major biopharmaceutical companies and academic institutions. The United States, in particular, is a dominant force, characterized by high adoption rates of advanced bioprinting technologies and a strong intellectual property landscape, fostering continuous innovation. Canada also contributes significantly through its research excellence and supportive funding for biomedical advancements.
- Europe: The European market holds a substantial share, propelled by a strong research base, increasing prevalence of chronic diseases necessitating regenerative therapies, and favorable government initiatives and funding programs supporting bioprinting and tissue engineering research across the continent. Countries such as Germany, the United Kingdom, France, and Italy are at the forefront of bioink research and application, benefiting from advanced healthcare systems and collaborative scientific networks that accelerate technological adoption and clinical translation.
- Asia Pacific (APAC): The Asia Pacific region is rapidly emerging as the fastest-growing market for bioinks, characterized by burgeoning healthcare expenditures, increasing medical tourism, and a growing awareness of advanced therapeutic options. Countries like China, Japan, South Korea, and Australia are witnessing significant investments in biotechnological research and development, coupled with a large and expanding patient pool. Government support for R&D, coupled with a focus on developing local expertise and manufacturing capabilities, is propelling the region's market expansion.
- Latin America: This region represents an emerging market for bioinks, demonstrating increasing investments in healthcare infrastructure and a growing interest in biotechnological research. While currently smaller in market share compared to developed regions, countries such as Brazil and Mexico are showing potential for growth due to improving economic conditions and a heightened focus on medical innovation. Strategic partnerships and technology transfer initiatives are key to unlocking the full potential of this nascent market.
- Middle East and Africa (MEA): The MEA region is a nascent market with significant future potential, driven by improving healthcare facilities, increasing government focus on medical innovation, and strategic collaborations with international research institutions and companies. The GCC countries (Saudi Arabia, UAE, etc.) are particularly active in investing in advanced healthcare technologies and research, aiming to diversify their economies and enhance their medical capabilities. Awareness and adoption of bioink technologies are steadily growing, positioning the region for future expansion.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Bioink Market.- CELLINK (BICO Group)
- Advanced BioMatrix, Inc.
- Allevi (3D Systems)
- Aspect Biosystems Ltd.
- Regenovo Biotechnology Co., Ltd.
- CollPlant Biotechnologies Ltd.
- Fluicell AB
- Organovo Holdings Inc.
- Rokit Healthcare
- Terasaki Institute for Biomedical Innovation (TIBI)
- EnvisionTEC GmbH
- GeSiM mbH
- Photocentric Ltd.
- BRIGHTCELLS, Inc.
- nScrypt Inc.
- MedPrin Regenerative Medical Technologies Co., Ltd.
- Eppendorf AG
- Merck KGaA
- Thermo Fisher Scientific Inc.
- Sigma-Aldrich (Merck KGaA)
Frequently Asked Questions
What are bioinks used for?
Bioinks are specialized biomaterials containing living cells and biomolecules, primarily used in 3D bioprinting to fabricate functional tissues and organs for applications in regenerative medicine, drug discovery, toxicology testing, and personalized medicine, by mimicking natural tissue architecture and function.
What materials are commonly found in bioinks?
Common bioink materials include natural polymers such as alginate, collagen, gelatin, and hyaluronic acid for their biocompatibility, and synthetic polymers like PLGA, PEG, and PCL for their tunable mechanical properties and degradation rates. Hybrid bioinks combine both types for optimized characteristics.
What are the main challenges in bioink development?
Key challenges in bioink development include balancing printability with high cell viability, achieving sufficient mechanical strength for functional tissues, ensuring proper vascularization for nutrient and waste exchange in large constructs, and navigating complex regulatory approval processes for clinical applications.
How does bioprinting contribute to regenerative medicine?
Bioprinting, leveraging bioinks, is crucial for regenerative medicine by enabling the precise fabrication of patient-specific tissues and organs, addressing donor organ shortages, and promoting the repair or replacement of damaged biological structures, leading to personalized and more effective therapeutic solutions.
What is the future outlook for the bioink market?
The bioink market is projected for significant growth, driven by continuous innovation in material science, advancements in bioprinting technologies, the increasing demand for personalized medicine, and expanding applications in drug discovery and therapeutic development, with AI playing an increasingly pivotal role in optimization.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
