Biological Safety Testing Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429257 | Date : Oct, 2025 | Pages : 255 | Region : Global | Publisher : MRU
Biological Safety Testing Market Size
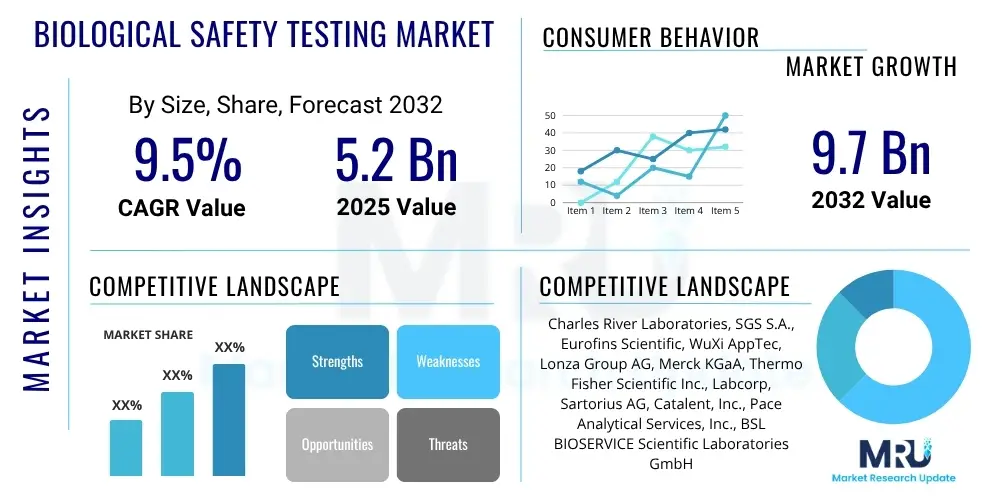
The Biological Safety Testing Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 9.5% between 2025 and 2032. The market is estimated at USD 5.2 Billion in 2025 and is projected to reach USD 9.7 Billion by the end of the forecast period in 2032.
Biological Safety Testing Market introduction
The Biological Safety Testing Market encompasses a critical suite of services and products essential for ensuring the safety, purity, and efficacy of biological products, including biopharmaceuticals, vaccines, and cell and gene therapies. These testing procedures are foundational to drug development and manufacturing, safeguarding against potential contamination from adventitious agents such as viruses, mycoplasma, bacteria, and endotoxins. The core objective is to prevent adverse effects in patients and ensure compliance with stringent global regulatory standards set by bodies like the FDA, EMA, and other national health authorities.
The primary offerings within this market include comprehensive testing services for raw materials, cell banks, bulk drug substances, and final drug products, alongside the provision of specialized kits, reagents, and instruments necessary for these analytical assays. Major applications span across the entire biopharmaceutical lifecycle, from early-stage research and development to commercial manufacturing and post-market surveillance. The intrinsic benefits of robust biological safety testing are manifold, centered on enhancing patient safety, mitigating product recall risks, and maintaining the integrity of pharmaceutical supply chains, which are paramount for public health.
Driving factors propelling this market's expansion include the escalating global demand for advanced biotherapeutics, a robust pipeline of novel biologics, and the increasing complexity of biological manufacturing processes. Furthermore, the persistent evolution and tightening of regulatory guidelines across all major pharmaceutical markets necessitate more rigorous and sophisticated safety testing protocols. The growing trend of outsourcing non-core activities to specialized contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) further contributes to market growth, as these entities often possess the advanced infrastructure and expertise required for intricate biological safety assessments.
Biological Safety Testing Market Executive Summary
The Biological Safety Testing Market is currently experiencing robust expansion, driven by significant advancements in biotechnology and an intensified focus on product safety within the biopharmaceutical industry. Key business trends indicate a strong move towards outsourcing specialized testing services to Contract Research Organizations (CROs) and Contract Development and Manufacturing Organizations (CDMOs), which are increasingly offering integrated solutions that encompass the full spectrum of biological safety requirements. Technological innovation is also a pivotal trend, with continuous development in rapid testing methods, automation, and advanced analytical platforms designed to enhance sensitivity, specificity, and throughput. Furthermore, consolidation activities, including mergers and acquisitions, are common as companies seek to expand their service portfolios, geographical reach, and technological capabilities, thereby strengthening their competitive positions within a highly specialized market.
Regionally, North America and Europe continue to dominate the market due to established biopharmaceutical industries, high research and development expenditures, and mature regulatory frameworks. However, the Asia Pacific (APAC) region is emerging as the fastest-growing market, propelled by increasing investments in biopharmaceutical R&D, rising healthcare expenditure, and a growing number of manufacturing facilities in countries like China, India, and South Korea. These nations are also becoming attractive hubs for outsourcing clinical trials and manufacturing, leading to a surge in demand for local biological safety testing services. Latin America and the Middle East & Africa regions are also showing nascent growth, driven by improving healthcare infrastructure and increasing awareness of biopharmaceutical product quality and safety.
Segment-wise, the market sees significant activity across various testing types and applications. Viral safety testing and cell line characterization remain critical segments, reflecting the foundational need to ensure the genetic stability and freedom from adventitious agents in biological products. The demand for sterility testing, mycoplasma detection, and endotoxin assays also continues to be high, integral to manufacturing processes for a wide range of biologics including vaccines, monoclonal antibodies, and gene therapies. Moreover, the gene and cell therapy sectors are experiencing exponential growth, leading to a burgeoning demand for specialized and highly sensitive biological safety tests tailored to these complex and innovative therapeutic modalities. These segments are characterized by the need for cutting-edge techniques and rigorous compliance to bring novel therapies to market safely.
AI Impact Analysis on Biological Safety Testing Market
User inquiries regarding the impact of Artificial Intelligence (AI) on the Biological Safety Testing Market frequently revolve around its potential to revolutionize efficiency, accuracy, and cost-effectiveness. Common questions explore how AI can accelerate testing protocols, enhance data interpretation, and improve predictive analysis for potential contaminants. Stakeholders are keen to understand if AI can reduce the human error factor, automate complex assays, and provide more robust insights into biological product safety profiles, ultimately streamlining regulatory submissions and reducing time-to-market for novel biotherapeutics. There is also significant interest in AI's role in addressing the challenges of complex gene and cell therapies, where traditional testing methods may be insufficient or time-consuming.
The integration of AI technologies is poised to significantly transform various facets of biological safety testing by introducing unparalleled levels of automation, precision, and analytical power. AI algorithms can be leveraged to analyze vast datasets generated from high-throughput screening, identify subtle patterns indicative of contamination, and predict potential safety issues with greater accuracy than conventional methods. This capability extends to image analysis for cell morphology studies, spectral analysis for impurity detection, and even sequence analysis for viral adventitious agent detection, thereby reducing the need for laborious manual interpretation and significantly shortening testing timelines.
Furthermore, AI-powered systems can optimize resource allocation within testing laboratories by predicting equipment maintenance needs, managing sample workflows, and even designing more efficient experimental protocols. This leads to a substantial reduction in operational costs and an increase in overall laboratory throughput, directly addressing key industry pain points. While the initial investment in AI infrastructure can be significant, the long-term benefits in terms of enhanced safety, regulatory compliance, and accelerated product development cycles are expected to drive widespread adoption across the biological safety testing landscape, making testing more reliable and accessible.
- Accelerated Data Analysis and Interpretation: AI algorithms process extensive datasets from various biological safety tests, including genomic sequencing, mass spectrometry, and cell-based assays, identifying subtle anomalies and contamination patterns far more rapidly and accurately than human analysts. This speeds up the detection of adventitious agents and streamlines risk assessment.
- Enhanced Predictive Modeling for Contamination Risk: Machine learning models can analyze historical data, manufacturing parameters, and raw material characteristics to predict the likelihood of contamination events, enabling proactive intervention and process optimization, thereby reducing costly failures and recalls.
- Automation of Routine and Complex Assays: AI drives advanced robotics and automation platforms for tasks such as sample preparation, aliquotting, and reagent dispensing, minimizing human error and variability. It also supports automated microscopy for cell line characterization and microbial detection, ensuring consistent and high-quality results.
- Improved Sensitivity and Specificity in Detection: AI-powered image analysis and signal processing techniques can enhance the sensitivity of diagnostic tests, enabling the detection of contaminants at lower concentrations or in earlier stages, which is crucial for highly potent and expensive biological products.
- Optimized Regulatory Compliance and Documentation: AI can assist in generating comprehensive audit trails, organizing complex documentation, and ensuring that testing protocols adhere strictly to evolving regulatory guidelines (e.g., FDA, EMA). This significantly reduces the burden of regulatory submissions and accelerates approval processes.
- Facilitation of Personalized Medicine and Gene Therapy Testing: For highly complex and personalized biological products like gene and cell therapies, AI provides the computational power to manage and interpret the unique safety profiles required, allowing for tailored testing strategies and more efficient quality control for these advanced therapeutic modalities.
- Reduction in Testing Costs and Time-to-Market: By streamlining workflows, automating tasks, and improving analytical precision, AI contributes to a significant reduction in the overall cost of biological safety testing and dramatically shortens the time required to bring safe biological products to market.
DRO & Impact Forces Of Biological Safety Testing Market
The Biological Safety Testing Market is profoundly shaped by a confluence of driving factors, restrictive challenges, and significant opportunities, alongside a dynamic interplay of impact forces. The primary drivers include the burgeoning global biologics market, characterized by an increasing pipeline of complex biopharmaceutical products such as monoclonal antibodies, vaccines, and cell and gene therapies, all of which necessitate stringent safety evaluations. Additionally, the tightening of regulatory frameworks by global health authorities, demanding exhaustive testing to ensure patient safety and product integrity, acts as a powerful catalyst for market expansion. The rising incidence of chronic and infectious diseases globally also fuels research and development in biologics, consequently elevating the demand for comprehensive biological safety testing services.
However, the market also faces considerable restraints, including the substantial cost associated with conducting advanced biological safety tests, which can be prohibitive for smaller biotech firms. The inherent complexity of regulatory landscapes across different regions, coupled with the need for highly specialized scientific expertise and advanced instrumentation, also poses significant challenges. Furthermore, the persistent shortage of skilled professionals capable of performing and interpreting these intricate tests can hinder market growth and limit the throughput of testing facilities, leading to bottlenecks in product development and release.
Opportunities within the market are vast and primarily reside in emerging economies, where healthcare infrastructure is rapidly developing, and access to advanced biotherapeutics is expanding. Technological advancements, such as the development of rapid, high-throughput, and cell-based assays, along with the integration of automation and artificial intelligence, present immense potential for improving efficiency and reducing testing timelines. The accelerating field of personalized medicine and the exponential growth of cell and gene therapy also open new avenues for specialized biological safety testing services, requiring novel approaches to ensure the safety of these highly innovative and potentially curative treatments. These emerging areas demand bespoke testing solutions and offer significant growth prospects for market players.
Segmentation Analysis
The Biological Safety Testing Market is comprehensively segmented to address the diverse needs of the biopharmaceutical industry, reflecting the breadth of products, technologies, and end-users involved. This segmentation allows for a granular understanding of market dynamics, enabling stakeholders to identify specific growth areas and tailor their strategies. Key segments include various product categories and service offerings, different types of tests conducted, the specific applications of biologics being tested, and the diverse end-user profiles that utilize these critical safety services. Each segment plays a vital role in the overall ecosystem, contributing to the assurance of product quality and patient safety throughout the biopharmaceutical development and manufacturing lifecycle.
- By Product & Service:
- Products
- Kits & Reagents: Encompasses consumable items such as media, buffers, detection kits (e.g., PCR kits, ELISA kits), and chemical reagents used in various biological safety assays.
- Instruments: Includes specialized laboratory equipment like real-time PCR systems, flow cytometers, automated microbial detection systems, and other analytical instruments essential for conducting biological safety tests.
- Services
- Cell Line Characterization: Testing to confirm the identity, purity, and genetic stability of cell lines used in bioproduction, ensuring they are free from adventitious agents and retain their desired characteristics.
- Viral Safety Testing: Comprehensive assays to detect known and unknown viral contaminants in cell banks, raw materials, and biological products, employing techniques like in vitro/in vivo assays, PCR, and NGS.
- Mycoplasma Detection: Highly sensitive tests to identify mycoplasma contamination, which can be difficult to detect and profoundly impact cell culture health and product quality.
- Sterility Testing: Assays to confirm the absence of viable microorganisms (bacteria, fungi) in sterile products, adhering to pharmacopeial requirements.
- Bioburden Testing: Quantification of the total number of viable microorganisms in raw materials, in-process samples, and non-sterile products, to ensure microbial control.
- Endotoxin Testing: Detection and quantification of bacterial endotoxins, which are pyrogenic substances from Gram-negative bacteria, using methods like LAL (Limulus Amebocyte Lysate) assay.
- Other Safety Tests: Includes a range of specialized tests such as adventitious agent testing, genetic stability testing, and host cell protein/DNA residual analysis.
- Products
- By Test Type:
- Sterility Tests: Ensures the absence of any living microorganisms in a pharmaceutical product.
- Bioburden Tests: Determines the total number of viable microorganisms present in a product or sample.
- Mycoplasma Tests: Detects the presence of mycoplasma contamination, critical for cell culture-derived products.
- Endotoxin Tests: Measures the level of bacterial endotoxins, potent pyrogens, in samples.
- Viral Safety Tests: Screens for and identifies potential viral contaminants in biological materials.
- Cell Line Authentication: Verifies the identity and purity of cell lines used in bioproduction.
- Other Test Types: Includes a variety of specialized assays such as specific pathogen testing.
- By Application:
- Vaccines: Testing for viral, bacterial, and other contaminants in vaccine production.
- Gene Therapy: Specialized safety testing for viral vectors, gene integrity, and potential oncogenicity.
- Monoclonal Antibodies: Extensive testing for purity, viral safety, and adventitious agents in antibody production.
- Recombinant Proteins: Safety assessments for proteins produced through recombinant DNA technology.
- Blood & Blood Products: Ensuring the viral and microbial safety of plasma-derived products and cellular components.
- Stem Cells: Characterization and safety testing for stem cell lines used in regenerative medicine.
- Other Biologics: Includes testing for various other complex biological products like therapeutic enzymes and tissue-engineered products.
- By End-User:
- Pharmaceutical & Biopharmaceutical Companies: Major consumers of biological safety testing services for their extensive pipelines and manufacturing operations.
- Contract Research Organizations (CROs) & Contract Development and Manufacturing Organizations (CDMOs): Providers of testing services for pharmaceutical clients, often specializing in complex or high-volume assays.
- Academic & Research Institutes: Utilize testing services for basic research, early-stage drug discovery, and development of novel biological tools.
- Medical Device Companies: Require testing for medical devices that come into contact with biological systems or contain biological components.
- Other End-Users: Includes governmental agencies, clinical laboratories, and environmental testing agencies.
Value Chain Analysis For Biological Safety Testing Market
The value chain for the Biological Safety Testing Market is complex and multi-faceted, involving several key stages from the initial sourcing of raw materials to the final delivery of testing results to end-users. At the upstream segment, the value chain begins with suppliers of critical raw materials, specialized reagents, analytical kits, and advanced instrumentation. These suppliers provide the foundational components necessary for performing biological safety assays, including cell culture media, biochemicals, antibodies, PCR master mixes, and sophisticated equipment such as real-time PCR machines, mass spectrometers, and automated robotic systems. The quality and reliability of these upstream inputs directly impact the accuracy and efficiency of subsequent testing procedures, making supplier relationships and quality control paramount.
Moving downstream, the core of the value chain involves the actual performance of biological safety testing services, which is typically carried out by specialized laboratories within pharmaceutical and biopharmaceutical companies, or by dedicated Contract Research Organizations (CROs) and Contract Development and Manufacturing Organizations (CDMOs). These entities employ highly skilled scientists and technicians, leverage advanced technological platforms, and operate under stringent quality management systems to conduct a wide array of tests, including viral safety, mycoplasma detection, sterility, endotoxin testing, and cell line characterization. The value added at this stage is significant, encompassing scientific expertise, regulatory compliance, data analysis, and the generation of comprehensive reports that are critical for product development and regulatory submissions.
Distribution channels in this market are primarily direct, especially for specialized services provided by CROs and CDMOs that engage directly with biopharmaceutical clients through service contracts and partnerships. However, for products like testing kits, reagents, and instruments, both direct and indirect channels are utilized. Direct sales involve manufacturers selling directly to end-users or through their own sales forces, offering technical support and specialized training. Indirect channels involve distributors and third-party logistics providers who facilitate broader market reach, particularly in geographically diverse regions. The complexity and highly regulated nature of biological safety testing often necessitate close collaboration between providers and customers, whether through direct engagement or through specialized distributors who can offer localized technical support and regulatory expertise, ensuring that the critical safety requirements of biological products are met throughout their lifecycle.
Biological Safety Testing Market Potential Customers
The potential customer base for the Biological Safety Testing Market is highly specialized and comprises entities deeply embedded within the biopharmaceutical and healthcare ecosystems, all with a shared imperative to ensure the safety and quality of biological products. The primary end-users are pharmaceutical and biopharmaceutical companies, which engage in extensive research, development, and manufacturing of biologics such as therapeutic proteins, vaccines, and advanced therapies. These companies rely heavily on biological safety testing to ensure their products are free from adventitious agents and comply with global regulatory standards, from early-stage cell line development through to commercial batch release. Their continuous pipeline of novel biological entities drives a consistent and growing demand for comprehensive testing services.
Another significant segment of potential customers includes Contract Research Organizations (CROs) and Contract Development and Manufacturing Organizations (CDMOs). These organizations provide specialized services, including biological safety testing, to pharmaceutical companies that choose to outsource portions of their R&D and manufacturing processes. CROs and CDMOs themselves become key buyers of sophisticated testing kits, reagents, and instruments, in addition to being direct providers of testing services. They often possess the advanced infrastructure, specialized expertise, and capacity to handle diverse testing requirements, making them critical partners in the biopharmaceutical value chain and substantial consumers within this market.
Furthermore, academic and research institutes, particularly those involved in biomedical research, drug discovery, and the development of new biological tools, represent a notable segment of potential customers. While their testing volumes may be lower than large pharmaceutical companies, they frequently require highly sensitive and innovative testing solutions for their cutting-edge projects, including cell and gene therapy research. Medical device companies, especially those developing devices that incorporate biological components or interact directly with human biological systems, also constitute a growing customer base. Lastly, governmental and public health organizations involved in surveillance, quality control, or regulatory oversight for biological products, also engage in or commission biological safety testing to ensure public health and safety standards are maintained across the industry.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 5.2 Billion |
| Market Forecast in 2032 | USD 9.7 Billion |
| Growth Rate | 9.5% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Charles River Laboratories, SGS S.A., Eurofins Scientific, WuXi AppTec, Lonza Group AG, Merck KGaA, Thermo Fisher Scientific Inc., Labcorp, Sartorius AG, Catalent, Inc., Pace Analytical Services, Inc., BSL BIOSERVICE Scientific Laboratories GmbH, GVK BIO, Bioreliance (Merck Millipore), Q2 Solutions (IQVIA), Syngene International Ltd., Sanofi Pasteur, Fujifilm Diosynth Biotechnologies, Avance Biosciences, Toxikon Corporation |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Biological Safety Testing Market Key Technology Landscape
The Biological Safety Testing Market is characterized by a rapidly evolving technological landscape, driven by the increasing complexity of biological products and the demand for faster, more accurate, and more sensitive detection methods. A foundational technology widely employed is Polymerase Chain Reaction (PCR) and its variants, such as quantitative PCR (qPCR) and reverse transcription PCR (RT-PCR), which are critical for the rapid and highly sensitive detection and identification of viral and bacterial nucleic acids. These molecular methods offer significant advantages over traditional cell-based assays in terms of speed and specificity, enabling quicker release of biological products and enhancing overall manufacturing efficiency. Advances in multiplex PCR allow for simultaneous detection of multiple contaminants, further streamlining testing workflows.
Another crucial technological pillar involves cell-based assays and immunology-based techniques like Enzyme-Linked Immunosorbent Assay (ELISA) and Western Blotting. Cell-based assays are essential for detecting viruses that may not be easily identified by molecular methods, as they provide a biologically relevant host system for viral replication and cytopathic effect observation. ELISA and similar immunochemical methods are vital for the detection of specific antigens, antibodies, and host cell impurities, providing quantitative data that is crucial for regulatory submissions. The development of more sensitive and high-throughput cell-based systems and automated ELISA platforms continues to push the boundaries of detection limits and assay consistency.
Furthermore, the market is increasingly adopting advanced genomic and proteomic technologies, including Next-Generation Sequencing (NGS) and Mass Spectrometry. NGS offers unparalleled capabilities for untargeted adventitious agent detection, allowing for the identification of unexpected viral or microbial contaminants without prior knowledge of their sequences. This technology is particularly valuable for novel therapies like gene and cell therapies where the risk profile of unknown agents can be higher. Mass spectrometry is used for detailed characterization of proteins, including host cell protein analysis and confirmation of product identity and purity. The integration of automation, robotics, and advanced bioinformatics tools to manage and interpret the vast amounts of data generated by these high-throughput technologies represents a significant trend, enhancing the efficiency, reliability, and cost-effectiveness of biological safety testing services, and moving the industry towards more comprehensive and holistic safety assessments.
Regional Highlights
- North America: This region maintains a dominant position in the Biological Safety Testing Market, primarily driven by a well-established biopharmaceutical industry, significant research and development investments, and the presence of numerous key market players and contract organizations. Stringent regulatory frameworks from agencies like the U.S. FDA, coupled with a high adoption rate of advanced testing technologies, ensure a consistent and high demand for comprehensive biological safety services. The region benefits from a robust ecosystem supporting biotechnology innovation, including strong academic institutions and venture capital funding, fostering a continuous pipeline of biologics requiring safety assessments.
- Europe: Europe represents a substantial market for biological safety testing, characterized by its mature pharmaceutical industry and a strong focus on regulatory compliance, particularly under the guidance of the European Medicines Agency (EMA). Countries like Germany, the UK, France, and Switzerland are major contributors, with significant R&D activities in biotechnology and a growing number of biomanufacturing facilities. The region's emphasis on quality and safety, combined with collaborations between academic institutions and industry, ensures a steady demand for sophisticated testing services, including those for advanced therapies.
- Asia Pacific (APAC): The APAC region is projected to be the fastest-growing market for biological safety testing, fueled by rapid economic development, increasing healthcare expenditure, and a burgeoning biopharmaceutical sector in countries such as China, India, Japan, and South Korea. These nations are becoming global hubs for pharmaceutical manufacturing and contract research, attracting significant foreign direct investment and fostering local innovation. The rising demand for biologics, coupled with improving regulatory standards and a growing emphasis on quality assurance, is accelerating the adoption of advanced biological safety testing services across the region.
- Latin America: This region is experiencing nascent but growing demand for biological safety testing, driven by increasing investments in healthcare infrastructure, expanding access to biopharmaceutical products, and a developing regulatory landscape. Countries like Brazil, Mexico, and Argentina are leading the charge, with growing local pharmaceutical production and a rising need for compliant safety testing. International collaborations and technology transfer are playing a crucial role in bringing advanced testing capabilities to the region, although market penetration is still lower compared to developed regions.
- Middle East & Africa (MEA): The MEA region presents emerging opportunities in the biological safety testing market. Growth is primarily driven by increasing awareness regarding biopharmaceutical product quality and safety, government initiatives to develop local healthcare and pharmaceutical manufacturing capabilities, and foreign investments in the biotechnology sector. While currently a smaller market, ongoing infrastructure development and a focus on expanding access to advanced medical treatments are expected to gradually increase the demand for biological safety testing services in select countries within this diverse region.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Biological Safety Testing Market.- Charles River Laboratories
- SGS S.A.
- Eurofins Scientific
- WuXi AppTec
- Lonza Group AG
- Merck KGaA
- Thermo Fisher Scientific Inc.
- Labcorp
- Sartorius AG
- Catalent, Inc.
- Pace Analytical Services, Inc.
- BSL BIOSERVICE Scientific Laboratories GmbH
- GVK BIO
- Bioreliance (Merck Millipore)
- Q2 Solutions (IQVIA)
- Syngene International Ltd.
- Sanofi Pasteur
- Fujifilm Diosynth Biotechnologies
- Avance Biosciences
- Toxikon Corporation
Frequently Asked Questions
Analyze common user questions about the Biological Safety Testing market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is biological safety testing and why is it essential?
Biological safety testing is a crucial set of analytical procedures performed on biological products, raw materials, and cell lines to detect and identify potential contaminants such as viruses, mycoplasma, bacteria, fungi, and endotoxins. It is essential because it ensures the safety, purity, and quality of biopharmaceuticals, vaccines, and cell therapies, protecting patient health and ensuring compliance with stringent global regulatory standards before products reach the market.
What are the main types of tests conducted in biological safety testing?
The primary types of tests conducted include viral safety testing (detecting adventitious and endogenous viruses), mycoplasma detection (identifying stealthy bacterial contaminants), sterility testing (confirming absence of viable microorganisms), endotoxin testing (quantifying pyrogenic bacterial components), bioburden testing (measuring microbial load), and cell line characterization (verifying identity, purity, and genetic stability of cell substrates).
How do regulatory bodies influence the biological safety testing market?
Regulatory bodies such as the FDA (U.S.), EMA (Europe), and PMDA (Japan) exert significant influence by establishing strict guidelines and requirements for the safety and quality of biological products. Their evolving mandates necessitate rigorous testing at every stage of development and manufacturing, driving demand for advanced testing services and ensuring market players adhere to the highest standards, thereby directly impacting market growth and technological adoption.
What is the role of Contract Research Organizations (CROs) in this market?
Contract Research Organizations (CROs) play a pivotal role by providing specialized biological safety testing services to biopharmaceutical companies, often acting as extensions of their in-house capabilities. CROs offer expertise, advanced infrastructure, and capacity for complex, high-volume, and niche testing, enabling pharmaceutical firms to focus on core competencies, reduce operational costs, and accelerate product development timelines while ensuring regulatory compliance.
What are the key technological trends shaping the future of biological safety testing?
Key technological trends include the increasing adoption of rapid molecular methods like quantitative PCR and Next-Generation Sequencing (NGS) for faster and more comprehensive contaminant detection. Automation and robotics are streamlining workflows, enhancing throughput, and reducing human error. Additionally, the integration of Artificial Intelligence (AI) and machine learning is emerging for advanced data analysis, predictive modeling, and process optimization, promising to revolutionize efficiency, accuracy, and cost-effectiveness in the market.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
