Breast Cancer Liquid Biopsy Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427294 | Date : Oct, 2025 | Pages : 248 | Region : Global | Publisher : MRU
Breast Cancer Liquid Biopsy Market Size
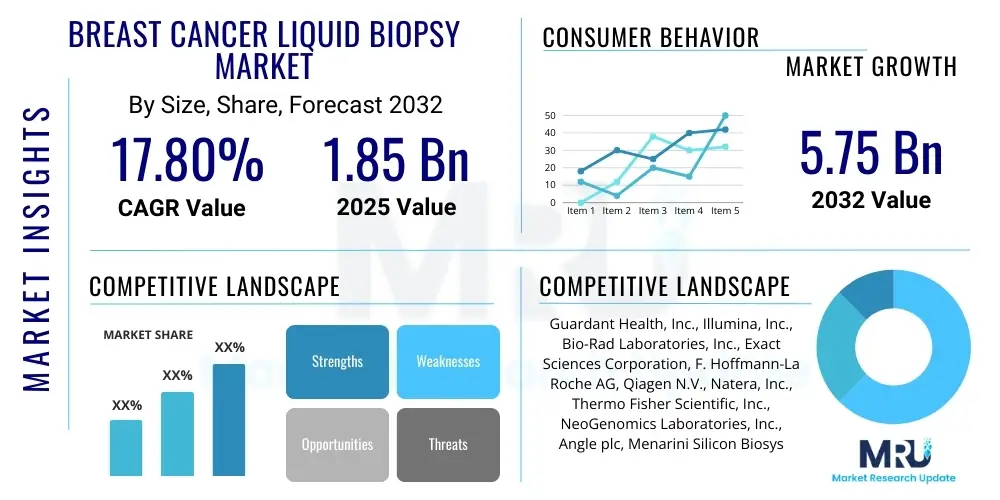
The Breast Cancer Liquid Biopsy Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 17.8% between 2025 and 2032. The market is estimated at USD 1.85 Billion in 2025 and is projected to reach USD 5.75 Billion by the end of the forecast period in 2032.
Breast Cancer Liquid Biopsy Market introduction
The Breast Cancer Liquid Biopsy Market is revolutionizing cancer diagnostics by offering a non-invasive alternative to traditional tissue biopsies. This innovative technology involves analyzing biological fluids, primarily blood, for circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), exosomes, and other tumor-derived components. Its primary applications span early detection, real-time disease monitoring, recurrence assessment, and guiding personalized treatment selection, offering significant advantages over invasive procedures which are often limited by tumor heterogeneity and sampling biases. The markets evolution is driven by a confluence of factors including the increasing global incidence of breast cancer, the imperative for earlier and more accurate diagnostic tools, and a growing demand for less invasive patient management strategies.
The inherent benefits of liquid biopsies, such as their minimally invasive nature, repeatability, and ability to capture tumor heterogeneity, position them as a pivotal advancement in oncology. They facilitate dynamic monitoring of treatment response, enabling clinicians to adjust therapeutic strategies promptly, thereby optimizing patient outcomes and reducing unnecessary treatments. Furthermore, the accessibility of liquid biopsy testing allows for frequent sampling, providing a longitudinal view of disease progression and resistance mechanisms that traditional biopsies cannot easily offer. This capability is particularly critical in metastatic breast cancer management, where monitoring for emergent resistance mutations is vital for sustained therapeutic efficacy.
Key driving factors propelling market expansion include significant technological advancements in molecular biology and genomic sequencing, such as Next-Generation Sequencing (NGS) and digital Polymerase Chain Reaction (dPCR), which enhance the sensitivity and specificity of detecting minute quantities of tumor-derived biomarkers. Additionally, increased research and development investments by pharmaceutical and biotechnology companies aimed at discovering novel biomarkers and developing more robust assay platforms are contributing to market growth. The rising geriatric population, which is more susceptible to breast cancer, alongside growing awareness among patients and healthcare professionals about the benefits of liquid biopsy, further underscores the markets robust growth trajectory and its transformative potential in breast cancer care.
Breast Cancer Liquid Biopsy Market Executive Summary
The Breast Cancer Liquid Biopsy Market is experiencing robust expansion, driven by significant advancements in molecular diagnostics and a pressing need for non-invasive, highly sensitive tools for cancer management. Business trends indicate a strong emphasis on strategic collaborations between diagnostic developers, pharmaceutical companies, and academic institutions to accelerate biomarker discovery, assay validation, and clinical integration. Investment in R&D remains a cornerstone, with a focus on enhancing assay sensitivity, specificity, and multiplexing capabilities to detect various tumor-derived analytes. Regulatory approvals and favorable reimbursement policies are gradually expanding, catalyzing market adoption, particularly in developed economies. The market is also witnessing a surge in mergers and acquisitions as larger players seek to consolidate expertise and expand their product portfolios, aiming to offer comprehensive diagnostic solutions from early detection to recurrence monitoring. Furthermore, a shift towards decentralized testing and point-of-care solutions is emerging, driven by the desire for quicker results and improved patient access, although centralized high-throughput labs currently dominate the landscape. Regional trends highlight North America and Europe as leading markets due to advanced healthcare infrastructure, significant R&D spending, and a high prevalence of breast cancer. However, the Asia-Pacific region is poised for the fastest growth, propelled by increasing healthcare expenditure, rising breast cancer incidence, and improving access to advanced diagnostic technologies. Within market segments, circulating tumor DNA (ctDNA) analysis currently holds the largest share due to its proven utility in mutation detection and monitoring, though circulating tumor cells (CTCs) and exosomes are gaining traction with ongoing research validating their clinical utility. Applications in therapy selection and recurrence monitoring are demonstrating the highest growth rates, reflecting the clinical imperative for personalized and dynamic patient management, while early detection remains a significant, albeit challenging, area of intense research focus.
AI Impact Analysis on Breast Cancer Liquid Biopsy Market
The integration of Artificial Intelligence (AI) and Machine Learning (ML) is poised to profoundly transform the Breast Cancer Liquid Biopsy Market, addressing critical challenges related to data interpretation, biomarker discovery, and diagnostic accuracy. Users frequently inquire about AIs potential to enhance the sensitivity and specificity of liquid biopsy assays, particularly in distinguishing true tumor signals from background noise and identifying complex genomic patterns indicative of early-stage disease or treatment resistance. There is significant interest in how AI can streamline the analysis of vast, multi-omic datasets generated by liquid biopsy platforms, moving beyond manual interpretation to reveal subtle, clinically actionable insights. Concerns often revolve around the validation of AI algorithms, the interpretability of AI-driven predictions, and the ethical implications of relying on automated systems for life-altering diagnoses, emphasizing the need for robust regulatory frameworks and transparent model development.
AIs role extends to accelerating the discovery and validation of novel biomarkers by sifting through complex biological data from clinical cohorts, identifying previously overlooked correlations between circulating analytes and disease states. This capability is crucial for expanding the utility of liquid biopsies beyond current established markers. Moreover, users are keen on AIs potential to improve patient stratification, predicting response to specific therapies with greater precision by analyzing a combination of liquid biopsy data, clinical parameters, and patient demographics. The ability of AI to learn from large datasets of patient outcomes and corresponding liquid biopsy profiles can lead to more refined risk assessments and personalized treatment recommendations, optimizing therapeutic strategies and potentially reducing adverse events.
Furthermore, AI algorithms are being explored for their capacity to enhance the efficiency and scalability of liquid biopsy testing by automating sample processing, data quality control, and report generation. This not only reduces human error but also enables higher throughput, making advanced diagnostics more accessible and cost-effective. The integration of AI also promises to facilitate real-time monitoring of disease progression and treatment efficacy, providing clinicians with immediate, data-driven insights to adapt patient management strategies dynamically. The overarching expectation is that AI will unlock the full potential of liquid biopsies, moving them from advanced research tools to indispensable, routine clinical instruments capable of delivering unparalleled accuracy and predictive power in breast cancer care.
- AI enhances the sensitivity and specificity of liquid biopsy assays through advanced pattern recognition.
- Facilitates the discovery of novel and complex biomarkers by analyzing multi-omic data.
- Improves diagnostic accuracy and early detection capabilities for breast cancer.
- Enables personalized treatment selection by predicting patient response to specific therapies.
- Automates data analysis, quality control, and report generation, increasing throughput and efficiency.
- Supports dynamic monitoring of disease progression and treatment resistance in real-time.
- Aids in identifying patients at higher risk of recurrence, allowing for timely intervention.
DRO & Impact Forces Of Breast Cancer Liquid Biopsy Market
The Breast Cancer Liquid Biopsy Market is significantly shaped by a dynamic interplay of Drivers, Restraints, Opportunities, and broader Impact Forces. Key drivers include the escalating global incidence of breast cancer, which necessitates innovative diagnostic and monitoring tools, alongside the inherent advantages of liquid biopsies such as their non-invasive nature, potential for early detection, and ability to track tumor heterogeneity and treatment response in real-time. Technological advancements in molecular diagnostics, particularly in Next-Generation Sequencing (NGS) and digital PCR, have dramatically improved assay sensitivity and specificity, further fueling market adoption. Increased research and development funding from both public and private sectors, coupled with a growing emphasis on personalized medicine, are also propelling market expansion. However, the market faces notable restraints, including the high cost associated with advanced liquid biopsy tests, which can be a barrier to widespread adoption, especially in resource-constrained regions. Regulatory complexities and the need for standardized protocols across different platforms and laboratories pose significant hurdles to market entry and broader clinical integration. Furthermore, challenges related to assay sensitivity and specificity, particularly in early-stage disease detection where circulating tumor markers are scarce, occasionally limit current clinical utility. Nevertheless, substantial opportunities exist, driven by the vast untapped potential in emerging economies with improving healthcare infrastructures, the development of multi-cancer early detection tests incorporating breast cancer markers, and the integration of artificial intelligence for enhanced data analysis and biomarker discovery. The advent of point-of-care liquid biopsy solutions could also dramatically expand market access and utility. Broader impact forces, such as evolving healthcare policies, changing reimbursement landscapes, and increasing public awareness regarding cancer screening, exert significant influence on market trajectory. The competitive intensity within the diagnostics sector, characterized by continuous innovation and strategic partnerships, also plays a crucial role in shaping the markets future, pushing for more effective, affordable, and accessible liquid biopsy solutions.
Segmentation Analysis
The Breast Cancer Liquid Biopsy Market is meticulously segmented across various parameters to provide a comprehensive understanding of its intricate dynamics and address diverse clinical needs. These segmentations are crucial for market players to tailor their strategies, identify niche opportunities, and develop targeted solutions that resonate with specific end-users and applications. The primary segmentation typically includes product type, biomarker type, technology, application, and end-user, each reflecting different facets of the markets current offerings and future growth potential. Understanding these segments is paramount for effective market penetration and for addressing the specific requirements of oncologists, diagnostic laboratories, and patients alike, ensuring that the development pipeline remains aligned with evolving clinical demands for precision diagnostics.
For instance, the product type segment differentiates between kits and reagents, instruments, and services, highlighting the varying business models and value propositions within the ecosystem. Kits and reagents represent the consumables essential for performing the assays, while instruments encompass the sophisticated platforms required for sample processing and analysis. Services, on the other hand, include the provision of testing by specialized diagnostic laboratories, often leveraging proprietary technologies and expertise. This differentiation helps in analyzing the revenue streams and competitive landscape for each component of the liquid biopsy workflow, from initial sample preparation to final result generation and interpretation.
Moreover, the segmentation by biomarker type (e.g., ctDNA, CTCs, exosomes, miRNAs) underscores the diverse biological targets being leveraged for breast cancer detection and monitoring, each with unique advantages and technical challenges. Similarly, technology segmentation (e.g., NGS, PCR, microarrays, mass spectrometry) reflects the underlying scientific methodologies employed, indicating the level of technological sophistication and analytical capability available in the market. Application-based segmentation (e.g., early detection, recurrence monitoring, therapy selection, prognosis) directly addresses the clinical utility of liquid biopsies at different stages of breast cancer management, while end-user segmentation (e.g., hospitals, diagnostic centers, research institutes) highlights the primary consumers of these advanced diagnostic tools, each having distinct purchasing patterns and integration requirements.
- By Product Type:
- Kits and Reagents
- Instruments
- Services
- By Biomarker Type:
- Circulating Tumor DNA (ctDNA)
- Circulating Tumor Cells (CTCs)
- Exosomes and Extracellular Vesicles (EVs)
- MicroRNAs (miRNAs)
- Other Biomarkers (e.g., proteins, methylation markers)
- By Technology:
- Next-Generation Sequencing (NGS)
- Polymerase Chain Reaction (PCR) based techniques (e.g., dPCR, qPCR)
- Microarray Technology
- Flow Cytometry
- Immunohistochemistry (IHC)
- Mass Spectrometry
- By Application:
- Early Detection and Screening
- Recurrence Monitoring
- Treatment Selection and Response Monitoring
- Prognosis and Risk Assessment
- Drug Discovery and Development
- By End-User:
- Hospitals and Clinics
- Diagnostic Laboratories and Reference Labs
- Research and Academic Institutions
- Pharmaceutical and Biotechnology Companies
Breast Cancer Liquid Biopsy Market Value Chain Analysis
The value chain for the Breast Cancer Liquid Biopsy Market encompasses a complex sequence of activities, beginning with fundamental research and extending to the ultimate delivery of diagnostic results to patients, involving multiple stakeholders. At the upstream stage, significant activities include basic research and development efforts in molecular biology, genomics, and oncology, often conducted by academic institutions, biotechnology startups, and pharmaceutical companies to discover novel biomarkers and validate their clinical utility. This phase also involves the development and manufacturing of specialized reagents, assays, and sophisticated instrumentation crucial for liquid biopsy testing. Key players here focus on enhancing the sensitivity, specificity, and throughput of their technologies to accurately detect rare tumor-derived analytes from patient samples, laying the groundwork for clinical application. Partnerships between research entities and technology providers are common to translate scientific discoveries into viable diagnostic products.
Moving downstream, the value chain involves the processing of patient samples, performance of the liquid biopsy assays, and the interpretation of the resulting data. This stage is primarily dominated by specialized diagnostic laboratories, reference labs, and increasingly, hospital pathology departments equipped with advanced molecular testing capabilities. These entities are responsible for adhering to stringent quality control standards, ensuring the accuracy and reliability of test results. The distribution channel plays a critical role in connecting the upstream technology providers with the downstream diagnostic service providers and ultimately, the end-users (clinicians and patients). Distribution can be direct, where technology manufacturers sell their instruments and kits directly to large diagnostic networks or hospitals, or indirect, involving third-party distributors who manage sales, logistics, and technical support across broader geographical regions. Indirect channels are particularly vital for market penetration in regions with less developed healthcare infrastructure or where local expertise is required to navigate regulatory environments and market nuances.
The final crucial steps involve the clinical interpretation of liquid biopsy results by oncologists and pathologists, followed by the integration of these insights into patient management strategies. The effectiveness of the entire value chain hinges on seamless collaboration, efficient information flow, and robust quality assurance mechanisms at every stage. Furthermore, the role of direct and indirect engagement with end-users is pivotal for market education, adoption, and feedback collection, which in turn influences future product development and service enhancements. Direct engagement often involves direct sales teams and medical science liaisons educating clinicians and lab personnel. Indirect engagement might involve professional societies, patient advocacy groups, and scientific conferences that disseminate knowledge and build confidence in liquid biopsy technologies, thereby influencing market demand and clinical practice guidelines.
Breast Cancer Liquid Biopsy Market Potential Customers
The Breast Cancer Liquid Biopsy Market caters to a diverse range of potential customers, all unified by the common objective of improving breast cancer patient outcomes through advanced diagnostics. Primarily, these include healthcare providers such as oncologists, surgeons, and general practitioners who seek non-invasive, highly informative tools for initial diagnosis, treatment planning, and long-term monitoring of their patients. Oncologists, in particular, are key decision-makers, relying on liquid biopsy results to guide personalized therapy selection, monitor therapeutic efficacy, detect early signs of recurrence, and identify resistance mechanisms. The ability of liquid biopsies to provide real-time insights into tumor dynamics without the need for repeated invasive procedures makes them highly attractive to clinicians looking to optimize patient management and enhance quality of life.
Beyond individual practitioners, major institutional buyers represent a significant segment of potential customers. These include hospitals and large healthcare systems that aim to integrate cutting-biopsy technologies into their comprehensive cancer care programs, often establishing specialized molecular diagnostics laboratories. Diagnostic laboratories and reference centers, both independent and hospital-affiliated, constitute another critical customer base. These entities purchase liquid biopsy kits, reagents, and instruments to offer testing services to clinicians and patients, often serving as central hubs for high-throughput analysis and expert interpretation. Their demand is driven by the need for reliable, scalable, and cost-effective solutions that can process a large volume of samples while maintaining high levels of accuracy and regulatory compliance.
Moreover, the market extends to research and academic institutions actively engaged in breast cancer research, biomarker discovery, and clinical trials. These customers utilize liquid biopsy platforms for translational research, understanding disease biology, identifying new therapeutic targets, and evaluating the efficacy of novel drugs. Pharmaceutical and biotechnology companies also represent a growing segment, employing liquid biopsies as companion diagnostics to stratify patients for clinical trials, monitor drug response, and assess the emergence of resistance in drug development pipelines. Their interest lies in leveraging liquid biopsies to accelerate drug development, streamline clinical trials, and ultimately bring more effective, targeted therapies to market. Each customer segment has specific needs regarding data integration, workflow efficiency, regulatory compliance, and cost-effectiveness, requiring market players to offer tailored solutions and support.
Breast Cancer Liquid Biopsy Market Key Technology Landscape
The Breast Cancer Liquid Biopsy Market is underpinned by a rapidly evolving and sophisticated technology landscape, characterized by continuous innovation aimed at enhancing the sensitivity, specificity, and multiplexing capabilities of diagnostic assays. At the forefront are advanced molecular techniques, predominantly Next-Generation Sequencing (NGS) and various Polymerase Chain Reaction (PCR) based methods, particularly digital PCR (dPCR). NGS offers unparalleled capabilities for comprehensive genomic profiling of circulating tumor DNA (ctDNA), enabling the detection of a wide array of genetic mutations, copy number variations, and fusion genes that are critical for personalized treatment selection and resistance monitoring. Its high throughput and ability to identify novel biomarkers make it indispensable for both clinical diagnostics and research. Digital PCR, on the other hand, excels in absolute quantification of rare mutations with extremely high sensitivity, making it ideal for detecting minimal residual disease or early recurrence where tumor-derived analytes are present in very low concentrations.
Beyond gene sequencing, other crucial technologies contribute to the broad utility of liquid biopsies. Microfluidics platforms are vital for the efficient isolation and enrichment of rare circulating tumor cells (CTCs) from peripheral blood, which are present in extremely low numbers among billions of normal blood cells. These platforms provide precise control over fluid dynamics at microscopic scales, enabling the capture, enumeration, and molecular characterization of intact CTCs, offering insights into metastatic potential and tumor biology. Mass spectrometry is gaining traction for the analysis of circulating proteins, metabolites, and other non-nucleic acid biomarkers, providing complementary information to genomic data and expanding the scope of liquid biopsy applications. Advances in immunoassay technologies are also crucial for the detection of specific protein markers associated with breast cancer, often employed in conjunction with genomic assays for a multi-modal approach to diagnostics.
Furthermore, the integration of computational biology and artificial intelligence (AI) is transforming the analysis and interpretation of the vast datasets generated by these technologies. AI algorithms are being developed to identify complex patterns, reduce noise, and extract clinically meaningful insights from genomic, proteomic, and cellular data, enhancing diagnostic accuracy and predictive power. Bioinformatic tools are essential for managing, analyzing, and interpreting the high-dimensional data, facilitating the identification of actionable mutations and the monitoring of disease progression. These technological advancements collectively contribute to the increasing reliability and clinical utility of breast cancer liquid biopsies, paving the way for more precise, less invasive, and patient-centric cancer management strategies, by continuously pushing the boundaries of what is detectable and interpretable from a simple blood draw.
Regional Highlights
- North America: This region dominates the Breast Cancer Liquid Biopsy Market, primarily driven by robust healthcare infrastructure, high research and development expenditures, early adoption of advanced diagnostic technologies, and the presence of leading market players. Favorable reimbursement policies, a high prevalence of breast cancer, and increasing awareness among healthcare professionals and patients further contribute to its leading position. The United States, in particular, is a hotbed of innovation and clinical trials, constantly pushing the boundaries of liquid biopsy applications.
- Europe: Europe represents a significant market share due to strong governmental support for cancer research, an aging population susceptible to breast cancer, and increasing investments in personalized medicine. Countries like Germany, the UK, and France are at the forefront of adopting liquid biopsy technologies, supported by well-established healthcare systems and a focus on integrating precision diagnostics into routine clinical practice. Regulatory harmonization efforts also facilitate market growth.
- Asia-Pacific: This region is projected to exhibit the highest growth rate during the forecast period, fueled by a rapidly expanding patient pool, improving healthcare infrastructure, and rising healthcare expenditures. Increased awareness about early cancer detection, growing adoption of advanced diagnostic techniques, and the presence of emerging economies like China and India investing heavily in healthcare modernization are key drivers. Strategic partnerships and local manufacturing initiatives are also accelerating market penetration.
- Latin America: The Latin American market is experiencing steady growth, driven by increasing breast cancer incidence, growing demand for non-invasive diagnostic procedures, and improving access to advanced medical technologies. Economic development and rising disposable incomes in countries like Brazil and Mexico are enabling greater investment in healthcare, although challenges related to reimbursement and infrastructure still exist. Awareness campaigns and international collaborations are helping to drive adoption.
- Middle East & Africa (MEA): The MEA region is an emerging market, with growth primarily attributed to increasing healthcare investments, a rising burden of cancer, and efforts to modernize diagnostic capabilities. Governments in countries like Saudi Arabia and UAE are actively promoting advanced medical technologies. However, market penetration remains lower compared to developed regions due to limited access to advanced facilities and varying healthcare policies, creating opportunities for targeted expansion by market players.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Breast Cancer Liquid Biopsy Market.- Guardant Health, Inc.
- Illumina, Inc.
- Bio-Rad Laboratories, Inc.
- Exact Sciences Corporation
- F. Hoffmann-La Roche AG (Roche Diagnostics)
- Qiagen N.V.
- Natera, Inc.
- Thermo Fisher Scientific, Inc.
- NeoGenomics Laboratories, Inc.
- Angle plc
- Menarini Silicon Biosystems
- Adaptive Biotechnologies Corporation
- Exosome Diagnostics (Bio-Techne)
- Personal Genome Diagnostics Inc. (PGDx)
- RainDance Technologies, Inc. (acquired by Bio-Rad)
Frequently Asked Questions
What is a liquid biopsy for breast cancer and how does it work?
A liquid biopsy for breast cancer is a non-invasive diagnostic test that analyzes tumor-derived material, such as circulating tumor DNA (ctDNA) or circulating tumor cells (CTCs), from a patients blood sample. It works by detecting genetic mutations, epigenetic changes, or protein markers released by cancer cells into the bloodstream, offering insights into the tumors characteristics without the need for an invasive tissue biopsy.
What are the main advantages of liquid biopsies over traditional tissue biopsies for breast cancer?
Liquid biopsies offer several key advantages: they are minimally invasive, reducing patient discomfort and risks; they can be repeated frequently for real-time monitoring of disease progression and treatment response; they capture tumor heterogeneity by sampling from multiple metastatic sites; and they are often more accessible for patients where a tissue biopsy might be difficult or impossible.
In which clinical applications are breast cancer liquid biopsies most commonly used?
Breast cancer liquid biopsies are increasingly used for monitoring treatment response, detecting early signs of recurrence, identifying resistance mutations to guide therapy adjustments, and stratifying patients for targeted therapies. While still under extensive research, their potential for early detection and screening of breast cancer is also a significant area of development.
What are the primary challenges limiting the widespread adoption of breast cancer liquid biopsies?
Key challenges include the high cost of the tests, which can impact reimbursement and patient access; the need for improved sensitivity and specificity, especially in early-stage disease where tumor markers are scarce; regulatory complexities for test validation and approval; and the requirement for standardization across different platforms and laboratories to ensure consistent and reliable results.
What is the future outlook for the Breast Cancer Liquid Biopsy Market?
The future outlook is highly promising, driven by continuous technological advancements, increasing research into novel biomarkers, and the integration of artificial intelligence for enhanced data analysis. The market is expected to expand significantly with growing adoption in early detection, personalized medicine, and long-term disease management, ultimately transforming breast cancer diagnosis and treatment paradigms.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
