Capnography Devices Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429473 | Date : Nov, 2025 | Pages : 251 | Region : Global | Publisher : MRU
Capnography Devices Market Size
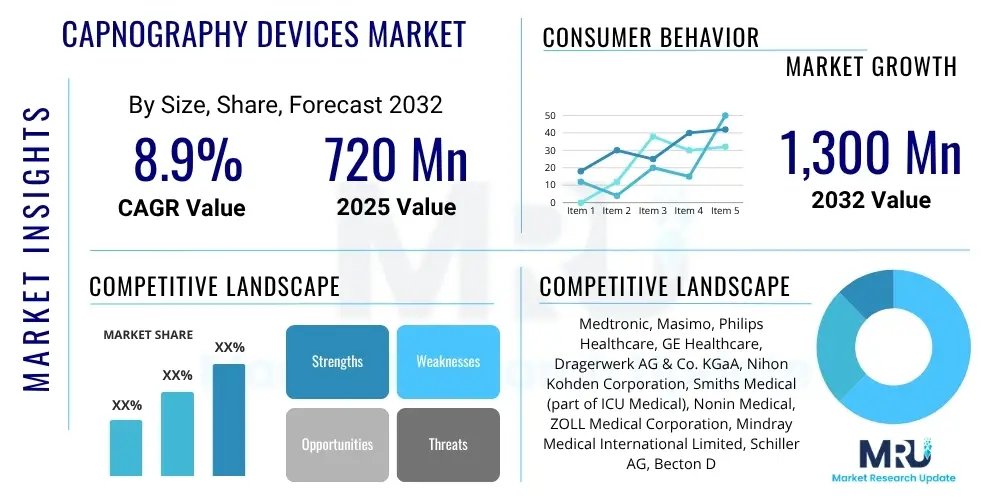
The Capnography Devices Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.9% between 2025 and 2032. The market is estimated at $720 million in 2025 and is projected to reach $1,300 million by the end of the forecast period in 2032.
Capnography Devices Market introduction
The Capnography Devices Market involves the production and distribution of medical equipment designed to monitor the concentration or partial pressure of carbon dioxide (CO2) in respiratory gases. These devices provide real-time, non-invasive measurements of end-tidal CO2 (EtCO2), which is crucial for assessing a patient's ventilatory status and confirming endotracheal tube placement. Capnography is considered a vital parameter in various clinical settings, offering immediate insights into circulatory and metabolic functions.
Products in this market range from standalone capnographs to integrated modules within multi-parameter patient monitors, available in mainstream, sidestream, and microstream technologies. Mainstream devices attach directly to the patient's airway, offering rapid response, while sidestream and microstream devices sample gases from the patient's breathing circuit via a small tube, suitable for patients with low tidal volumes. The primary applications span critical care units, operating rooms during anesthesia, emergency departments, and pre-hospital settings, where monitoring respiratory status is paramount for patient safety.
The benefits of capnography are substantial, including early detection of respiratory depression, hypercapnia, and hypocapnia, verification of intubation, and assessment of cardiopulmonary resuscitation (CPR) effectiveness. Key driving factors for market growth include the increasing number of surgical procedures globally, a rising prevalence of chronic respiratory diseases such as COPD and asthma, and a growing emphasis on patient safety standards in healthcare. Technological advancements leading to more portable, accurate, and user-friendly devices further contribute to market expansion.
Capnography Devices Market Executive Summary
The Capnography Devices Market is poised for significant expansion, driven by continuous innovation and an increasing global focus on enhancing patient safety in diverse medical environments. Business trends indicate a shift towards the integration of capnography into comprehensive patient monitoring systems, alongside the development of more compact, portable, and wireless devices that cater to an expanding range of clinical applications, including pre-hospital and home care settings. Manufacturers are also focusing on improving the accuracy and response time of these devices, while reducing their overall cost to facilitate broader adoption.
Regional trends reveal North America currently dominating the market, attributed to its advanced healthcare infrastructure, high adoption rate of sophisticated medical technologies, and stringent patient safety guidelines. Europe also holds a substantial share, propelled by an aging population and robust healthcare spending. However, the Asia Pacific region is projected to exhibit the fastest growth, primarily due to improving healthcare access, rising medical tourism, increasing awareness of advanced monitoring techniques, and a growing patient pool suffering from respiratory and chronic diseases. Latin America, the Middle East, and Africa are also showing promising growth as healthcare infrastructure develops.
Segment trends highlight the continued dominance of quantitative capnography due to its precision and reliability, particularly in critical applications like anesthesia and intensive care. Among product types, mainstream capnography devices maintain a strong position for their direct measurement capabilities and quick response. However, sidestream and microstream technologies are gaining traction, especially in non-intubated patients and settings requiring lower sample flow rates. The end-user landscape sees hospitals remaining the largest segment, though ambulatory surgical centers and pre-hospital emergency care are rapidly increasing their adoption of these essential monitoring tools.
AI Impact Analysis on Capnography Devices Market
User inquiries regarding AI's influence on the Capnography Devices Market frequently center on how artificial intelligence can augment existing capabilities, particularly concerning data interpretation, predictive analytics, and integration with broader healthcare systems. Common themes include the potential for AI to enhance diagnostic accuracy by identifying subtle patterns in EtCO2 waveforms, predict adverse respiratory events before they become critical, and streamline clinical workflows through automated analysis. There is also significant interest in AI's role in integrating capnography data with electronic health records (EHRs) and other patient monitoring systems to create a more holistic view of patient health, addressing concerns about data overload and the need for more actionable insights. Users are keen to understand the practical applications, the level of reliability, and the potential for improved patient outcomes, while also expressing caution regarding data privacy, algorithmic bias, and the regulatory challenges associated with deploying AI in critical medical devices.
- Enhanced Diagnostic Interpretation: AI algorithms can analyze complex capnography waveforms and trends, identifying subtle anomalies that may indicate specific respiratory or circulatory conditions more rapidly and accurately than human interpretation alone.
- Predictive Analytics for Adverse Events: AI can leverage real-time and historical capnography data, combined with other physiological parameters, to predict the onset of respiratory distress, cardiac arrest, or other critical events, enabling earlier intervention.
- Optimized Workflow and Alert Systems: AI-powered capnography devices can integrate with hospital information systems to automate data logging, reduce false alarms, and provide context-rich alerts, thereby improving clinician efficiency and reducing alarm fatigue.
- Personalized Patient Monitoring: AI can tailor monitoring parameters and alert thresholds based on individual patient characteristics, clinical history, and treatment plans, leading to more personalized and effective care.
- Support for Remote and Telemedicine Applications: AI can facilitate the remote monitoring of capnography data, providing automated analysis and flagging critical changes for remote clinicians, thereby extending the reach of specialized respiratory care.
DRO & Impact Forces Of Capnography Devices Market
The Capnography Devices Market is significantly influenced by a dynamic interplay of driving forces, restraining factors, and emerging opportunities. A primary driver is the global increase in the number of surgical procedures, which inherently necessitates robust respiratory monitoring to ensure patient safety during anesthesia and post-operative recovery. Concurrently, the rising prevalence of chronic respiratory diseases, such as Chronic Obstructive Pulmonary Disease (COPD) and asthma, particularly among the aging population, is fueling the demand for continuous and accurate ventilation monitoring devices, both in hospital and home care settings. Furthermore, a heightened global emphasis on improving patient safety standards and reducing adverse events in healthcare environments is compelling healthcare providers to adopt advanced monitoring solutions like capnography, which offers immediate and reliable physiological data.
Despite these strong drivers, the market faces several notable restraints. The relatively high cost associated with advanced capnography devices can be a significant barrier to adoption, especially in developing economies or healthcare facilities with limited budgets. This financial constraint often leads to slower integration of these technologies compared to regions with higher healthcare expenditures. Additionally, a lack of widespread awareness and adequate training regarding the benefits and proper use of capnography devices among healthcare professionals in certain regions can hinder market penetration. Stringent regulatory approval processes for medical devices also pose challenges for manufacturers, leading to extended development timelines and increased costs to bring new innovations to market, thereby potentially delaying wider availability and adoption.
Nevertheless, the market is rich with opportunities that promise future growth and innovation. Emerging markets across Asia Pacific, Latin America, and the Middle East and Africa present considerable untapped potential due to rapidly improving healthcare infrastructure and increasing healthcare expenditure. The development of portable and handheld capnography devices represents a significant opportunity, catering to pre-hospital emergency care, home care settings, and remote clinics, expanding accessibility beyond traditional hospital environments. Furthermore, the integration of capnography with telemedicine platforms and the advent of AI-powered solutions offer avenues for enhanced diagnostics, predictive analytics, and streamlined patient management, revolutionizing how respiratory health is monitored and managed. These technological advancements and geographical expansions are expected to mitigate current restraints and propel the market forward.
Segmentation Analysis
The Capnography Devices Market is broadly segmented based on various critical parameters, including product type, technology, application, end-user, and portability. Each segmentation provides a detailed lens through which market dynamics, preferences, and growth trajectories can be analyzed, offering insights into niche demands and overarching industry shifts. Understanding these segments is crucial for stakeholders to tailor strategies, develop targeted products, and identify high-growth areas within the diverse landscape of respiratory monitoring solutions. The market exhibits distinct growth patterns and competitive landscapes across its different segments, driven by technological advancements, evolving clinical needs, and regional healthcare policies.
- Product Type
- Mainstream Capnography Devices: These devices directly attach to the patient's airway adapter, providing immediate, breath-by-breath EtCO2 readings. They are known for their rapid response time and accuracy.
- Sidestream Capnography Devices: These devices sample gas from the patient's breathing circuit via a narrow tube, drawing a small continuous sample into the monitor for CO2 analysis. They are versatile for both intubated and non-intubated patients.
- Microstream Capnography Devices: A specialized type of sidestream technology that uses a very low sample flow rate, making them ideal for neonates, pediatric patients, and those with low tidal volumes.
- Technology
- Qualitative Capnography: Primarily used for verifying endotracheal tube placement, providing a colorimetric or visual change to indicate the presence of CO2, without precise numerical values.
- Quantitative Capnography: Provides precise numerical values and waveform displays of CO2 concentration, offering detailed insights into ventilatory, circulatory, and metabolic status. This is the predominant technology in advanced clinical settings.
- Application
- Critical Care: Used in intensive care units (ICUs) for continuous monitoring of critically ill patients, helping manage ventilation and detect respiratory compromise.
- Emergency Medicine: Essential in emergency departments and pre-hospital settings for rapid assessment of respiratory status, intubation confirmation, and monitoring during resuscitation.
- Pain Management: Employed during procedural sedation for pain management procedures to monitor respiratory depression caused by analgesics and sedatives.
- Procedural Sedation: Utilized during conscious sedation for various medical procedures to ensure patient safety by monitoring respiratory effort and EtCO2 levels.
- Diagnostic Imaging: Used to monitor patient ventilation during MRI or CT scans, especially for sedated patients or those requiring controlled breathing.
- Other Applications: Includes applications in dentistry, sleep labs, and home care.
- End User
- Hospitals: The largest end-user segment, including operating rooms, ICUs, emergency rooms, and general wards.
- Ambulatory Surgical Centers (ASCs): Increasingly adopting capnography for monitoring patients undergoing outpatient surgeries and procedures under sedation.
- Pre-hospital Emergency Care: Emergency medical services (EMS) personnel utilize portable capnographs for patient assessment and monitoring during transport.
- Home Care Settings: Growing adoption for patients with chronic respiratory conditions requiring continuous monitoring, often with portable devices.
- Portability
- Portable Devices: Lightweight, battery-operated devices designed for use in various locations, including pre-hospital care, patient transport, and home care.
- Standalone Devices: Dedicated capnographs that are typically larger and designed for fixed use in hospital settings like operating rooms and ICUs.
Value Chain Analysis For Capnography Devices Market
The value chain for the Capnography Devices Market encompasses a series of interconnected stages, beginning with research and development and extending through manufacturing, distribution, and end-user application. Upstream activities primarily involve the procurement of raw materials and specialized components essential for device construction. This includes sourcing high-precision sensors, microcontrollers, display units, and other electronic components from a global network of suppliers. Manufacturers then engage in the intricate process of assembling these components, developing proprietary software, and conducting rigorous testing to ensure product accuracy, reliability, and compliance with medical device standards. The quality and availability of these specialized components significantly impact production efficiency and the final product's performance, forming a critical foundation for the entire value chain.
Midstream activities focus on the manufacturing, assembly, and quality control of capnography devices. This stage involves sophisticated engineering to integrate various technologies, such as infrared spectroscopy for CO2 detection, with user interfaces and data processing capabilities. Manufacturers often invest heavily in research and development to innovate new features, improve accuracy, reduce size, and enhance user experience, thereby differentiating their products in a competitive market. Strict adherence to regulatory requirements, such as FDA approvals and CE markings, is paramount during this stage, ensuring devices meet global safety and performance benchmarks before they can enter the market. The efficiency of manufacturing processes directly affects the cost-effectiveness and scalability of device production, influencing market accessibility.
Downstream activities involve the distribution, sales, and post-sales support of capnography devices to end-users. Distribution channels are varied and include direct sales forces, third-party distributors, and value-added resellers. Direct sales allow manufacturers to maintain close relationships with large hospital networks and key opinion leaders, while indirect channels provide broader market reach, particularly in diverse geographical regions. Both direct and indirect distribution strategies are crucial for market penetration. End-users, such as hospitals, ambulatory surgical centers, and emergency medical services, purchase these devices and rely on ongoing technical support, maintenance, and training. Effective post-sales service is vital for customer satisfaction and maintaining market reputation. The final stage involves the actual clinical application of the devices, where their benefits in patient monitoring and safety are realized, completing the value chain and informing future product development through feedback loops.
Capnography Devices Market Potential Customers
The primary end-users and potential customers for capnography devices are diverse and span various segments within the healthcare ecosystem, all driven by the need for enhanced patient safety and real-time respiratory monitoring. Hospitals represent the largest segment of potential customers, particularly their operating rooms, intensive care units (ICUs), emergency departments, and recovery rooms. In these critical environments, capnography is indispensable for monitoring patients under anesthesia, those requiring mechanical ventilation, and individuals experiencing acute respiratory distress. The increasing volume of surgical procedures and the focus on reducing post-operative complications continually drive demand from hospital settings, making them central to market growth.
Ambulatory Surgical Centers (ASCs) constitute another significant and rapidly growing customer base. As more minor surgeries and diagnostic procedures shift from inpatient to outpatient settings, ASCs require reliable monitoring equipment to ensure patient safety during procedural sedation and recovery. The demand for portable and cost-effective capnography solutions is particularly strong within ASCs. Similarly, pre-hospital emergency care providers, including ambulance services and emergency medical technicians (EMTs), are increasingly adopting portable capnographs. These devices are crucial for verifying endotracheal tube placement in the field, monitoring ventilation during patient transport, and assessing the effectiveness of cardiopulmonary resuscitation (CPR) before hospital arrival, greatly enhancing pre-hospital care capabilities.
Beyond acute care settings, other potential customers include specialized clinics such as pain management clinics, dental offices performing conscious sedation, and sleep disorder centers. There is also a burgeoning market in home care settings, driven by the rising prevalence of chronic respiratory diseases and the increasing trend towards remote patient monitoring. Patients with conditions like COPD or sleep apnea, who require continuous or intermittent respiratory assessment, represent a growing segment for more user-friendly, non-invasive capnography devices. Educational institutions and research laboratories also form a niche customer segment for training and scientific investigation, further broadening the market's reach.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | $720 million |
| Market Forecast in 2032 | $1,300 million |
| Growth Rate | 8.9% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Medtronic, Masimo, Philips Healthcare, GE Healthcare, Dragerwerk AG & Co. KGaA, Nihon Kohden Corporation, Smiths Medical (part of ICU Medical), Nonin Medical, ZOLL Medical Corporation, Mindray Medical International Limited, Schiller AG, Becton Dickinson and Company (BD), Skanray Technologies Pvt. Ltd., Infinium Medical, EMERGE Inc., Diamedica (UK) Limited, Utas Co. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Capnography Devices Market Key Technology Landscape
The Capnography Devices Market is characterized by a dynamic and evolving technological landscape, with continuous innovations aimed at improving accuracy, portability, and integration capabilities. A cornerstone technology is infrared (IR) spectroscopy, specifically non-dispersive infrared (NDIR) technology, which forms the basis for measuring CO2 concentration by detecting its absorption of specific IR wavelengths. Advancements in NDIR have led to more compact, robust, and energy-efficient sensors, allowing for smaller and more portable capnograph designs. These technological improvements are crucial for enabling real-time, breath-by-breath analysis with high precision, which is critical in acute care and emergency settings where rapid and reliable data is essential for clinical decision-making.
Beyond the core sensing technology, significant progress has been made in the development of mainstream, sidestream, and microstream sampling techniques. Microstream technology, in particular, has seen improvements, offering ultra-low sample flow rates that are ideal for non-intubated patients, neonates, and those with very small tidal volumes, minimizing the impact on patient respiration. This precision allows for effective monitoring across a broader patient population. Furthermore, the integration of capnography modules into multi-parameter patient monitors is a key trend, providing clinicians with a comprehensive view of physiological data on a single screen, streamlining workflows and enhancing overall patient assessment. This integration leverages advanced data processing algorithms to correlate capnography data with other vital signs, offering deeper diagnostic insights.
The advent of wireless connectivity and wearable sensor technologies is further transforming the market. Wireless capnographs facilitate greater patient mobility and reduce clutter in clinical environments, while enabling remote monitoring capabilities that are increasingly vital for home care and telemedicine applications. Miniaturization efforts are leading to ultra-portable and even disposable capnography solutions, opening up new opportunities in pre-hospital, austere, and mass casualty environments. The nascent integration of artificial intelligence (AI) and machine learning (ML) algorithms is also emerging as a significant technological development. These AI-powered systems can analyze complex capnography waveforms, identify subtle trends, predict respiratory deterioration, and provide smart alerts, thereby augmenting clinical decision-making and potentially preventing adverse events by providing more proactive and data-driven insights into patient conditions.
Regional Highlights
- North America: This region holds the largest market share, driven by a highly developed healthcare infrastructure, high adoption rates of advanced medical technologies, and a strong emphasis on patient safety protocols. The presence of major market players, substantial healthcare expenditure, and a high volume of surgical procedures further contribute to its dominance. The United States and Canada are key contributors to the regional market due to favorable reimbursement policies and robust R&D activities.
- Europe: Europe represents a significant market, propelled by an aging population susceptible to chronic respiratory diseases, stringent regulatory frameworks promoting patient safety, and widespread adoption of advanced medical devices. Countries such as Germany, the UK, France, and Italy are leading the market with high healthcare spending and continuous technological advancements in patient monitoring solutions.
- Asia Pacific (APAC): Expected to be the fastest-growing region, owing to improving healthcare infrastructure, increasing healthcare expenditure, a large patient pool with respiratory conditions, and rising awareness of advanced monitoring techniques. Emerging economies like China, India, Japan, and South Korea are investing heavily in modernizing their healthcare systems, offering significant growth opportunities for capnography device manufacturers.
- Latin America: This region is experiencing steady growth, supported by increasing investments in healthcare infrastructure, growing awareness of advanced patient monitoring, and an expanding medical tourism sector. Brazil and Mexico are key markets due to their relatively larger economies and improving access to medical technologies.
- Middle East and Africa (MEA): The MEA market is gradually expanding, primarily driven by improving healthcare facilities, increasing government initiatives to enhance healthcare services, and a rising prevalence of chronic diseases. Countries like Saudi Arabia, UAE, and South Africa are at the forefront of adopting modern medical equipment, including capnography devices, to elevate patient care standards.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Capnography Devices Market.- Medtronic
- Masimo
- Philips Healthcare
- GE Healthcare
- Dragerwerk AG & Co. KGaA
- Nihon Kohden Corporation
- Smiths Medical (part of ICU Medical)
- Nonin Medical
- ZOLL Medical Corporation
- Mindray Medical International Limited
- Schiller AG
- Becton Dickinson and Company (BD)
- Skanray Technologies Pvt. Ltd.
- Infinium Medical
- EMERGE Inc.
- Diamedica (UK) Limited
- Utas Co.
Frequently Asked Questions
Analyze common user questions about the Capnography Devices market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is capnography and why is it important in medical care?
Capnography is a monitoring technique that measures the concentration of carbon dioxide in exhaled breath. It is crucial because it provides real-time, non-invasive insights into a patient's ventilatory status, circulatory function, and metabolic activity, essential for patient safety during anesthesia, critical care, and emergency situations.
What are the main types of capnography devices available?
The primary types of capnography devices include mainstream, sidestream, and microstream capnographs. Mainstream devices offer direct, rapid measurements at the airway, while sidestream and microstream devices sample gases, with microstream being ideal for patients with low tidal volumes or non-intubated individuals.
How is artificial intelligence impacting the Capnography Devices Market?
AI is enhancing capnography by enabling more accurate waveform analysis, predictive analytics for adverse respiratory events, and seamless integration with other patient data. This leads to improved diagnostics, streamlined clinical workflows, and more proactive patient management, optimizing outcomes and reducing clinician burden.
What are the key factors driving the growth of the capnography market?
Key drivers include the increasing number of surgical procedures, a rising global prevalence of chronic respiratory diseases, and a heightened focus on patient safety standards in healthcare. Technological advancements leading to more portable and user-friendly devices also significantly contribute to market expansion.
Which regions are leading in the adoption and growth of capnography devices?
North America currently leads the market due to its advanced healthcare infrastructure and high adoption rates. Europe also holds a strong position. However, the Asia Pacific region is projected for the fastest growth, driven by improving healthcare access and increasing medical expenditure.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
