Cardiovascular Clinical Trials Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 431318 | Date : Nov, 2025 | Pages : 258 | Region : Global | Publisher : MRU
Cardiovascular Clinical Trials Market Size
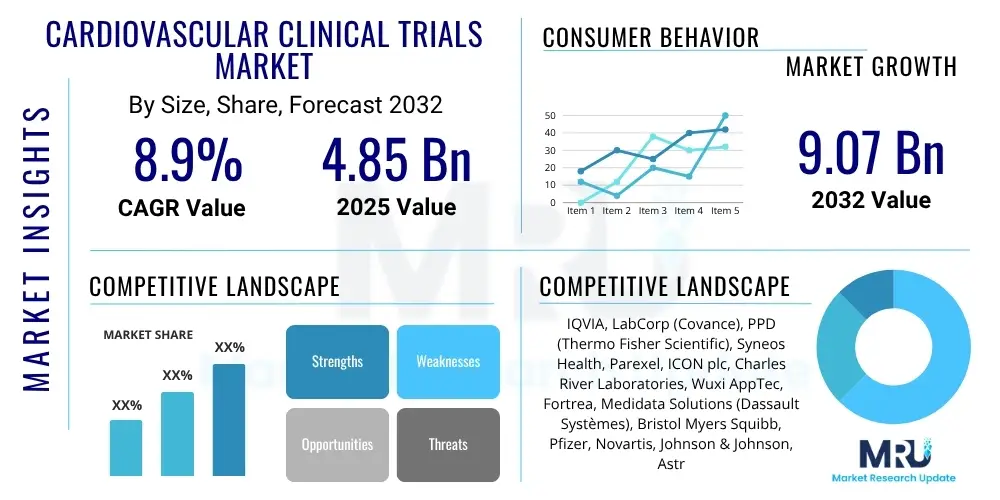
The Cardiovascular Clinical Trials Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.9% between 2025 and 2032. The market is estimated at USD 4.85 billion in 2025 and is projected to reach USD 9.07 billion by the end of the forecast period in 2032.
Cardiovascular Clinical Trials Market introduction
The Cardiovascular Clinical Trials Market encompasses research studies designed to evaluate the safety and efficacy of new drugs, medical devices, and treatment strategies for cardiovascular diseases (CVDs). These trials are critical for advancing medical science, introducing innovative therapies, and improving patient outcomes in a field burdened by high mortality and morbidity rates. Products evaluated range from novel pharmacological agents and biologics to advanced interventional devices like stents, pacemakers, and heart valves, as well as digital health solutions aimed at prevention and management.
Major applications of cardiovascular clinical trials include the development of treatments for conditions such as coronary artery disease, heart failure, arrhythmias, hypertension, and peripheral artery disease. These trials are essential for obtaining regulatory approval for new interventions, allowing them to reach patients globally. The benefits of a robust clinical trial ecosystem are profound, leading to a continuous influx of advanced therapeutic options that enhance quality of life and extend lifespan for millions affected by CVDs.
The market is primarily driven by the escalating global prevalence of cardiovascular diseases, an aging population more susceptible to heart conditions, significant advancements in medical technology, and increased research and development investments by pharmaceutical and medical device companies. Furthermore, a growing emphasis on evidence-based medicine and personalized treatment approaches fuels the demand for rigorous clinical investigation.
Cardiovascular Clinical Trials Market Executive Summary
The Cardiovascular Clinical Trials Market is experiencing robust growth driven by a convergence of factors, including the rising global burden of cardiovascular diseases, an aging demographic, and continuous innovation in medical science. Business trends indicate a shift towards more complex and specialized trials, increased outsourcing to Contract Research Organizations (CROs), and the adoption of decentralized trial models leveraging digital technologies to enhance efficiency and patient engagement. Significant investments in research and development by pharmaceutical and biotechnology firms, alongside medical device manufacturers, are fueling market expansion.
Regionally, North America and Europe continue to dominate the market due to well-established research infrastructure, substantial healthcare spending, and a high concentration of leading pharmaceutical and biotech companies. However, the Asia Pacific region is emerging as a significant growth hub, attracted by large patient populations, lower operational costs, and developing regulatory frameworks. Latin America and the Middle East & Africa are also showing potential, with increasing healthcare investments and a growing number of clinical trial initiatives.
Segment-wise, drug development trials for novel cardiovascular therapeutics remain a cornerstone, while device trials for innovative implants and interventional procedures are steadily expanding. The market is also witnessing growth in early-phase trials, emphasizing biomarker identification and precision medicine approaches. Furthermore, the increasing integration of artificial intelligence and real-world evidence solutions is optimizing trial design, patient recruitment, and data analysis, contributing to more efficient and impactful research outcomes.
AI Impact Analysis on Cardiovascular Clinical Trials Market
Users frequently inquire about artificial intelligence's potential to revolutionize cardiovascular clinical trials, focusing on how AI can accelerate drug discovery, optimize trial design, enhance patient recruitment and monitoring, and improve data analysis. Common concerns revolve around data privacy, ethical considerations, the validation of AI algorithms, and the need for robust regulatory frameworks. There is a strong expectation that AI will lead to more personalized, efficient, and cost-effective trials, ultimately bringing life-saving treatments to patients faster, while also addressing the complexities associated with large datasets and complex biological interactions within cardiovascular diseases.
- AI accelerates drug discovery by identifying potential therapeutic targets and predicting drug efficacy.
- Optimizes trial design through predictive analytics, patient stratification, and adaptive trial methodologies.
- Enhances patient recruitment by analyzing electronic health records (EHRs) to identify eligible candidates.
- Improves patient monitoring via wearable devices and remote sensors, generating real-time data for better safety and efficacy assessment.
- Facilitates advanced data analysis and interpretation of vast datasets, including imaging, genomic, and clinical data.
- Reduces operational costs and timelines by streamlining various trial processes, from protocol development to regulatory submission.
- Enables precision medicine by identifying patient subgroups most likely to respond to specific therapies.
- Mitigates human error in data collection and analysis, leading to more accurate and reliable trial results.
DRO & Impact Forces Of Cardiovascular Clinical Trials Market
The Cardiovascular Clinical Trials Market is profoundly shaped by a dynamic interplay of drivers, restraints, and opportunities. Key drivers include the overwhelming global prevalence of cardiovascular diseases, necessitating continuous development of new treatments, and the substantial increase in research and development expenditure by pharmaceutical and medical device companies. The aging global population, which is more prone to cardiovascular conditions, also significantly contributes to the demand for advanced therapies. Furthermore, technological advancements in diagnostics, imaging, and data analytics tools enhance the capabilities and efficiency of trials.
However, the market faces significant restraints, such as the exceedingly high cost associated with conducting cardiovascular clinical trials, which can span hundreds of millions of dollars per drug or device. The protracted timelines for trials, often extending over many years, present substantial financial and operational challenges. Difficulties in patient recruitment and retention, particularly for rare conditions or highly specific patient profiles, and stringent regulatory requirements across different geographies, further impede market growth. Ethical considerations surrounding patient safety and data privacy also add layers of complexity.
Despite these challenges, numerous opportunities exist to propel the market forward. The integration of advanced technologies like AI, machine learning, and real-world evidence (RWE) offers avenues for optimizing trial design, improving patient selection, and accelerating data analysis. The growing trend of decentralized clinical trials (DCTs), leveraging telemedicine and remote monitoring, enhances patient convenience and access while potentially reducing costs and improving diversity. The increasing focus on precision medicine, tailored to individual patient genetic profiles, opens new therapeutic frontiers. Lastly, the expansion into emerging markets provides access to larger, diverse patient populations and potential cost efficiencies.
Segmentation Analysis
The Cardiovascular Clinical Trials Market is broadly segmented based on various factors that define the scope and nature of these critical research endeavors. These segmentations provide a granular understanding of market dynamics, revealing key areas of growth, investment, and technological innovation. Categorizations typically include the phase of the trial, the specific therapeutic area being addressed, the type of study conducted, the sponsor funding the research, and the end-use settings where trials are performed.
Understanding these segments is vital for stakeholders, including pharmaceutical companies, medical device manufacturers, contract research organizations, and academic institutions, to strategically allocate resources, identify market niches, and tailor their research approaches. The complexity and duration of trials vary significantly across these segments, influencing costs, regulatory pathways, and ultimately, the market landscape. Each segment represents a distinct challenge and opportunity within the expansive field of cardiovascular research and development.
- By Phase:
- Phase I Clinical Trials
- Phase II Clinical Trials
- Phase III Clinical Trials
- Phase IV Clinical Trials (Post-marketing Surveillance)
- By Therapeutic Area:
- Coronary Artery Disease (CAD)
- Heart Failure
- Arrhythmia
- Hypertension
- Valvular Heart Disease
- Peripheral Artery Disease (PAD)
- Cardiomyopathy
- Congenital Heart Defects
- Stroke
- Other Cardiovascular Diseases
- By Type of Study:
- Interventional Studies
- Observational Studies
- Expanded Access Studies
- By Sponsor:
- Pharmaceutical & Biopharmaceutical Companies
- Medical Device Companies
- Contract Research Organizations (CROs)
- Academic & Research Institutions
- Government Agencies
- Other Sponsors
- By End-use:
- Hospitals
- Specialty Clinics
- Research Centers
- Ambulatory Surgical Centers
Value Chain Analysis For Cardiovascular Clinical Trials Market
The value chain for the Cardiovascular Clinical Trials Market is intricate, involving numerous specialized stages and diverse stakeholders, each contributing to the successful development and commercialization of new cardiovascular therapies. Upstream activities begin with extensive drug discovery and preclinical research, where potential compounds or devices are identified and rigorously tested in laboratory settings and animal models before human trials commence. This phase often involves pharmaceutical companies, biotechnology firms, and specialized research laboratories, laying the foundational science for clinical investigation. Contract Research Organizations (CROs) and Site Management Organizations (SMOs) play crucial roles in facilitating and managing these early stages.
Midstream activities primarily focus on the actual execution of clinical trials, spanning Phase I through Phase IV. This involves protocol development, regulatory submissions, patient recruitment, clinical site management, data collection, monitoring, and quality assurance. This phase is heavily dependent on collaboration between sponsors (pharmaceutical, biotech, or medical device companies), CROs, clinical investigators, and institutional review boards (IRBs). Data management and biostatistics are critical components, ensuring data integrity and statistical validity of results, often outsourced to specialized vendors.
Downstream activities involve the comprehensive analysis of trial data, preparation of detailed clinical study reports, and submission of these reports to regulatory bodies for market approval. Following approval, Phase IV trials (post-marketing surveillance) monitor long-term safety and efficacy, while commercialization and distribution strategies are implemented. The distribution channel for cardiovascular therapies is primarily direct from manufacturers to healthcare providers, but indirect channels through distributors, wholesalers, and pharmacies are also vital in ensuring patient access. The entire chain emphasizes quality, compliance, and strategic partnerships to navigate the complex landscape of clinical development.
Cardiovascular Clinical Trials Market Potential Customers
The primary potential customers and end-users within the Cardiovascular Clinical Trials Market are entities actively engaged in the research, development, and commercialization of new treatments for cardiovascular diseases. These include pharmaceutical and biotechnology companies that are constantly innovating new drug candidates and biologics to address unmet medical needs in cardiology. Medical device manufacturers represent another significant customer segment, as they require rigorous clinical testing for novel cardiovascular implants, diagnostic tools, and interventional devices, such as stents, pacemakers, and heart valves, to secure regulatory approvals and demonstrate clinical utility.
Furthermore, academic research institutions and university hospitals are crucial customers, often conducting investigator-initiated trials or collaborating with industry sponsors on groundbreaking research. These entities contribute significantly to the scientific advancement and knowledge base of cardiovascular medicine. Government agencies and public health organizations also initiate or fund clinical trials, especially those focused on public health issues, disease prevention, or comparative effectiveness research, serving as important indirect customers by creating demand for trial services.
Lastly, Contract Research Organizations (CROs) themselves can be considered both service providers and, in some contexts, customers, when they subcontract specialized services like central lab testing, medical imaging, or data management to niche vendors. Ultimately, the market serves the broader healthcare ecosystem, aiming to bring safer and more effective cardiovascular therapies to patients globally, with patients being the ultimate beneficiaries of successful clinical trials.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 4.85 Billion |
| Market Forecast in 2032 | USD 9.07 Billion |
| Growth Rate | 8.9% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | IQVIA, LabCorp (Covance), PPD (Thermo Fisher Scientific), Syneos Health, Parexel, ICON plc, Charles River Laboratories, Wuxi AppTec, Fortrea, Medidata Solutions (Dassault Systèmes), Bristol Myers Squibb, Pfizer, Novartis, Johnson & Johnson, AstraZeneca, Eli Lilly, Amgen, Merck & Co., Roche, Sanofi |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Cardiovascular Clinical Trials Market Key Technology Landscape
The Cardiovascular Clinical Trials Market is increasingly leveraging a sophisticated array of technologies to enhance efficiency, accuracy, and patient engagement, driving significant advancements in research methodologies. Electronic Data Capture (EDC) systems remain foundational, enabling secure and centralized collection of clinical trial data, reducing paper-based errors, and accelerating data management processes. Complementary to EDC are Clinical Trial Management Systems (CTMS), which provide comprehensive tools for managing project timelines, budgets, resources, and regulatory documents, ensuring trials run smoothly and efficiently from initiation to close-out.
Integration with Electronic Health Records (EHRs) is becoming paramount, allowing for more streamlined patient identification, eligibility screening, and longitudinal data collection, which significantly improves the relevance and depth of trial data. The advent of wearable devices and remote monitoring technologies has transformed patient data collection, enabling continuous and real-time physiological measurements (e.g., heart rate, blood pressure, ECG) outside traditional clinical settings. This not only enhances patient convenience but also provides a more complete picture of patient health and response to treatment in their natural environment, especially crucial for cardiovascular conditions.
Furthermore, the application of Artificial Intelligence (AI) and Machine Learning (ML) analytics is rapidly expanding, from optimizing trial design and identifying suitable patient cohorts to predicting trial outcomes and accelerating data interpretation. Telemedicine and decentralized trial (DCT) platforms facilitate remote patient consultations, virtual site visits, and remote consent processes, improving patient accessibility and retention, particularly in geographically dispersed or underserved populations. Genomic sequencing and advanced biomarker analysis are also critical, enabling precision medicine approaches that tailor treatments based on individual genetic predispositions, holding immense promise for cardiovascular care.
Regional Highlights
- North America: This region consistently holds the largest share of the Cardiovascular Clinical Trials Market, driven by robust research and development infrastructure, significant healthcare expenditure, high prevalence of cardiovascular diseases, and the presence of numerous leading pharmaceutical, biotechnology, and medical device companies. The U.S. in particular benefits from a well-established regulatory framework, advanced technological adoption, and substantial funding for clinical research.
- Europe: Europe represents a strong second market, characterized by extensive academic research, a large aging population susceptible to CVDs, and a strong emphasis on medical innovation. Countries like the UK, Germany, France, and Switzerland are key players, supported by sophisticated healthcare systems and a collaborative research environment, although navigating diverse national regulatory landscapes can pose challenges.
- Asia Pacific (APAC): The APAC region is projected to exhibit the highest growth rate, primarily due to its vast and diverse patient pool, rising prevalence of cardiovascular diseases, improving healthcare infrastructure, and lower operational costs compared to Western counterparts. Countries such as China, India, Japan, and South Korea are becoming attractive destinations for clinical trials, fostering both local and international research initiatives.
- Latin America: This region is experiencing steady growth, offering a diverse patient population and increasing investments in healthcare infrastructure. Countries like Brazil, Mexico, and Argentina are emerging as important locations for cardiovascular clinical trials, benefiting from a growing pool of experienced investigators and a relatively lower cost of conducting trials.
- Middle East and Africa (MEA): While currently a smaller market, MEA presents significant growth potential. Increasing healthcare spending, a growing burden of chronic diseases including CVDs, and efforts to modernize regulatory frameworks are attracting more clinical research activities to countries like Saudi Arabia, UAE, and South Africa.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Cardiovascular Clinical Trials Market.- IQVIA
- LabCorp (Covance)
- PPD (Thermo Fisher Scientific)
- Syneos Health
- Parexel
- ICON plc
- Charles River Laboratories
- Wuxi AppTec
- Fortrea
- Medidata Solutions (Dassault Systèmes)
- Bristol Myers Squibb
- Pfizer
- Novartis
- Johnson & Johnson
- AstraZeneca
- Eli Lilly and Company
- Amgen
- Merck & Co.
- Roche Holding AG
- Sanofi S.A.
Frequently Asked Questions
What are cardiovascular clinical trials?
Cardiovascular clinical trials are research studies that evaluate the safety and efficacy of new drugs, medical devices, and treatment approaches for heart and blood vessel conditions, aiming to improve patient outcomes and introduce innovative therapies into medical practice.
How does AI impact cardiovascular clinical trials?
AI significantly impacts cardiovascular clinical trials by optimizing trial design, accelerating patient recruitment, enhancing real-time monitoring through wearables, and improving the analysis of complex data, leading to faster, more efficient, and personalized development of therapies.
What are the biggest challenges in these trials?
The biggest challenges include the high cost of conducting trials, protracted timelines, difficulties in recruiting and retaining diverse patient populations, and navigating stringent regulatory requirements across different global regions.
Which regions are leading in cardiovascular clinical research?
North America, particularly the United States, and Europe are currently leading in cardiovascular clinical research due to advanced infrastructure, high R&D investments, and established regulatory systems. Asia Pacific is emerging as a rapidly growing region.
What is the future outlook for the Cardiovascular Clinical Trials Market?
The future outlook for the Cardiovascular Clinical Trials Market is positive, driven by continued innovation, the integration of digital technologies like AI and decentralized trial models, and a growing focus on precision medicine, all contributing to more effective and patient-centric research.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
