Cardiovascular Devices Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427938 | Date : Oct, 2025 | Pages : 253 | Region : Global | Publisher : MRU
Cardiovascular Devices Market Size
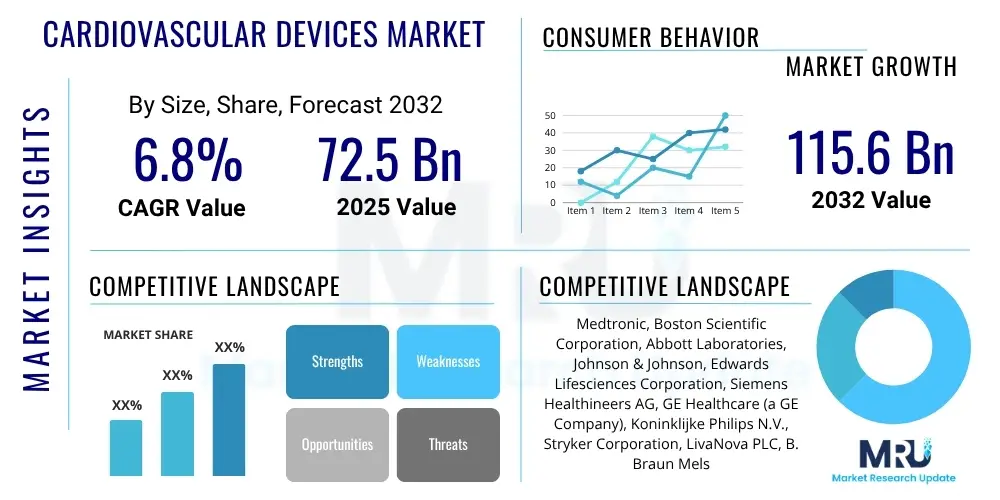
The Cardiovascular Devices Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2025 and 2032. The market is estimated at $72.5 Billion USD in 2025 and is projected to reach $115.6 Billion USD by the end of the forecast period in 2032.
Cardiovascular Devices Market introduction
The Cardiovascular Devices Market encompasses a vast array of medical technologies designed for the diagnosis, monitoring, and treatment of various cardiovascular diseases (CVDs). These devices range from advanced imaging systems and minimally invasive surgical tools to life-sustaining implants like pacemakers and artificial heart valves. The primary objective of these innovations is to improve patient outcomes, enhance quality of life, and extend longevity for individuals suffering from heart and vascular conditions. The continuous evolution of these devices addresses the pressing global health challenge posed by CVDs, which remain a leading cause of mortality worldwide.
Major applications of cardiovascular devices span across numerous medical disciplines, including cardiology, interventional cardiology, cardiac surgery, and vascular surgery. These applications include the diagnosis of complex heart conditions through echocardiography and electrophysiology studies, the treatment of coronary artery blockages with stents and angioplasty balloons, the management of cardiac arrhythmias with implantable cardioverter-defibrillators (ICDs), and the repair or replacement of diseased heart valves. Benefits derived from these devices are profound, leading to reduced recovery times, fewer complications, and more effective long-term disease management, often enabling patients to return to active, fulfilling lives.
Several critical factors are driving the robust growth of this market. The escalating global prevalence of cardiovascular diseases, largely attributed to an aging population, sedentary lifestyles, and increasing rates of diabetes and obesity, creates a sustained demand for effective diagnostic and therapeutic solutions. Concurrently, rapid technological advancements, including the development of smaller, more sophisticated, and more durable devices, along with the integration of artificial intelligence and advanced imaging, are continuously expanding the capabilities and reach of cardiovascular interventions. Furthermore, rising healthcare expenditures, increased awareness of early detection, and supportive government initiatives promoting heart health also play significant roles in propelling market expansion.
Cardiovascular Devices Market Executive Summary
The Cardiovascular Devices Market is experiencing dynamic growth, driven by an aging global population, the rising incidence of chronic cardiovascular diseases, and continuous technological innovation. Business trends indicate a strong focus on minimally invasive procedures, personalized medicine, and the integration of digital health solutions, including remote monitoring and AI-powered diagnostics. Companies are increasingly investing in research and development to create next-generation devices that offer greater efficacy, safety, and patient comfort, while also expanding into emerging markets to capitalize on untapped growth potential. Strategic partnerships, mergers, and acquisitions are also prevalent, aimed at consolidating market share, diversifying product portfolios, and gaining access to novel technologies.
Regionally, North America continues to dominate the market due to its advanced healthcare infrastructure, high healthcare spending, and favorable reimbursement policies. However, the Asia Pacific region is rapidly emerging as a significant growth hub, fueled by increasing awareness, improving healthcare access, a large patient pool, and growing medical tourism. Europe maintains a strong presence, characterized by a focus on innovation and established regulatory frameworks, while Latin America and the Middle East & Africa show promising growth trajectories as healthcare systems evolve and investments increase. These regional dynamics highlight a global shift towards broader accessibility and advanced care delivery for cardiovascular conditions.
Segment trends within the market reveal strong performance across various device categories. Therapeutic and surgical devices, particularly stents, catheters, and heart valves, command a substantial share due to their critical role in interventional cardiology and cardiac surgery. Diagnostic and monitoring devices are also experiencing significant growth, driven by the increasing emphasis on early detection and preventative care, alongside the adoption of portable and remote monitoring technologies. The shift towards less invasive procedures and devices that offer improved long-term outcomes is a prominent trend influencing development and adoption across all segments, underscoring the market's commitment to patient-centric care and technological advancement.
AI Impact Analysis on Cardiovascular Devices Market
The integration of Artificial Intelligence (AI) into the Cardiovascular Devices Market is transforming various facets, from diagnostics and treatment planning to surgical execution and post-operative care. Users frequently inquire about how AI can enhance the accuracy of cardiac imaging, predict disease progression, and personalize treatment regimens. There is considerable interest in AI's role in interpreting vast amounts of patient data from wearable devices and electronic health records to identify early warning signs and improve risk stratification. Concerns often revolve around data privacy, regulatory hurdles for AI-powered devices, the potential for algorithmic bias, and the need for robust validation to ensure clinical safety and efficacy. Expectations are high for AI to reduce diagnostic errors, streamline clinical workflows, and ultimately lead to more precise and effective cardiovascular interventions.
AI's influence is evident in several key areas, including automated image analysis for conditions like coronary artery disease and valvular heart disease, enabling faster and more accurate diagnoses. It plays a crucial role in developing predictive models for cardiac events, allowing for proactive intervention strategies. Furthermore, AI is being leveraged to optimize device performance, such as in adaptive pacing for pacemakers or intelligent control systems for robotic-assisted surgeries, enhancing precision and reducing procedural risks. The ability of AI to analyze complex physiological data also facilitates the development of personalized treatment plans, tailoring therapies to individual patient needs and responses. This technological advancement promises to revolutionize the standard of care in cardiovascular medicine, offering unprecedented levels of insight and operational efficiency.
- Enhanced diagnostic accuracy and speed through AI-powered image analysis (e.g., echocardiograms, CT scans, MRIs).
- Personalized treatment plans and drug dosages based on AI analysis of patient data and genetic profiles.
- Predictive analytics for early detection of cardiac events and disease progression, improving preventative care.
- Optimization of surgical procedures through AI-assisted robotics, enhancing precision and reducing invasiveness.
- Development of smart, adaptive cardiovascular implants (e.g., pacemakers, defibrillators) with AI-driven functionality.
- Real-time monitoring and data interpretation from wearable and remote devices for continuous patient management.
- Streamlined R&D processes for new cardiovascular devices and therapies, accelerating drug discovery and device innovation.
- Improved clinical trial design and patient recruitment through AI-driven data analysis.
- Reduction in healthcare costs through increased efficiency, reduced readmissions, and optimized resource allocation.
DRO & Impact Forces Of Cardiovascular Devices Market
The Cardiovascular Devices Market is shaped by a complex interplay of Drivers, Restraints, and Opportunities, collectively forming its Impact Forces. Key drivers include the ever-increasing global prevalence of cardiovascular diseases, propelled by an aging population and lifestyle-related risk factors such as obesity and diabetes. This demographic shift inherently increases the patient pool requiring diagnostic, therapeutic, and monitoring devices. Furthermore, significant technological advancements, including the development of more sophisticated, minimally invasive, and AI-integrated devices, continuously expand treatment options and improve patient outcomes, fueling market growth. Supportive government initiatives and increased public awareness campaigns focused on early detection and prevention of heart conditions also contribute to heightened demand for these devices, making them indispensable components of modern healthcare systems.
However, the market also faces notable restraints that can impede its growth trajectory. The high cost associated with advanced cardiovascular devices and surgical procedures remains a significant barrier, particularly in developing economies with limited healthcare budgets and less comprehensive reimbursement policies. Stringent regulatory frameworks and lengthy approval processes, especially in major markets like the U.S. and Europe, can delay market entry for innovative products, increasing R&D costs and time-to-market. Additionally, the shortage of skilled healthcare professionals capable of performing complex cardiovascular procedures and managing advanced device technologies presents a workforce challenge, impacting the adoption and effective utilization of these devices. These factors necessitate careful strategic planning by manufacturers and healthcare providers.
Despite these challenges, substantial opportunities exist for market expansion and innovation. Emerging economies, particularly in Asia Pacific and Latin America, represent largely untapped markets with rapidly improving healthcare infrastructures and growing disposable incomes, promising significant growth potential. The increasing adoption of remote patient monitoring and telecardiology solutions, especially accelerated by recent global health crises, presents a lucrative avenue for market players to develop connected health ecosystems for chronic disease management. Moreover, continued research into personalized medicine, regenerative therapies, and advanced biomaterials for device manufacturing offers promising future avenues, leading to devices that are more biocompatible, durable, and tailored to individual patient needs. These opportunities encourage investment and innovation, shaping the future landscape of cardiovascular care.
Segmentation Analysis
The Cardiovascular Devices Market is comprehensively segmented based on various factors, including device type, application, and end-use, to provide a detailed understanding of its diverse landscape. This segmentation allows for a granular analysis of market dynamics, identifying key growth areas and competitive advantages within specific product categories and therapeutic fields. The distinctions in device types reflect the wide range of technological sophistication and clinical utility, from simple diagnostic tools to complex implantable devices. Similarly, categorizing by application highlights the prevalence and treatment approaches for different cardiovascular conditions, while end-use segmentation underscores the primary settings where these devices are utilized, offering insights into healthcare delivery models and purchasing behaviors.
- Device Type:
- Diagnostic & Monitoring Devices
- ECG Devices
- Holter Monitors
- Event Monitors
- Stress Test Systems
- Cardiac Output Monitors
- Blood Pressure Monitors
- Pulse Oximeters
- Echocardiographs
- Doppler Ultrasound
- Cardiac Biomarker Test Kits
- Therapeutic & Surgical Devices
- Catheters (Diagnostic Catheters, Angiography Catheters, Electrophysiology Catheters)
- Stents (Drug-Eluting Stents, Bare-Metal Stents, Bioresorbable Stents)
- Guidewires
- Balloons (Angioplasty Balloons)
- Pacemakers (Single-Chamber, Dual-Chamber, Biventricular)
- Defibrillators (Internal/Implantable Cardioverter-Defibrillators (ICDs), External Defibrillators)
- Artificial Heart Valves (Mechanical Heart Valves, Bioprosthetic Heart Valves, Transcatheter Heart Valves)
- Heart-Lung Machines
- Annuloplasty Rings
- Bypass Grafts
- Ablation Devices (RF Ablation, Cryoablation)
- Clot Retrieval Devices
- Prosthetic Devices
- Ventricular Assist Devices (VADs)
- Artificial Hearts
- Diagnostic & Monitoring Devices
- Application:
- Coronary Artery Disease
- Cardiac Arrhythmias
- Heart Failure
- Peripheral Artery Disease
- Hypertension
- Congenital Heart Defects
- Other Cardiovascular Conditions
- End-Use:
- Hospitals & Clinics
- Ambulatory Surgical Centers (ASCs)
- Diagnostic Centers
- Home Care Settings
Value Chain Analysis For Cardiovascular Devices Market
The value chain for the Cardiovascular Devices Market is a complex ecosystem involving multiple stages, from raw material sourcing to end-user delivery and post-market support. The upstream segment of this chain is characterized by a reliance on specialized raw material suppliers providing biocompatible polymers, metals, and advanced ceramics, along with component manufacturers producing microelectronics, sensors, and intricate mechanical parts. These suppliers often require specialized expertise and adhere to stringent quality and regulatory standards due to the critical nature of medical devices. Robust research and development activities, often involving collaborations with academic institutions and clinical experts, are also integral to the upstream process, driving innovation and the creation of next-generation devices.
The core of the value chain involves the design, manufacturing, and assembly of the cardiovascular devices themselves, typically performed by large medical device companies. This stage requires significant capital investment in highly specialized manufacturing facilities, advanced automation, and rigorous quality control processes to ensure product safety and efficacy. Following manufacturing, devices move into the distribution phase, which can involve both direct and indirect channels. Direct distribution involves manufacturers selling directly to hospitals, clinics, and surgical centers through their own sales forces, allowing for greater control over product messaging, training, and customer relationships. This approach is often favored for high-value or highly specialized devices requiring extensive technical support.
Indirect distribution, on the other hand, relies on third-party distributors, wholesalers, and group purchasing organizations (GPOs) to reach a broader customer base, particularly for widely used or standardized devices. These intermediaries play a crucial role in logistics, warehousing, and local market penetration, especially in regions where manufacturers may not have a direct presence. The downstream segment of the value chain focuses on the end-users, primarily hospitals, cardiac catheterization labs, ambulatory surgical centers, and diagnostic clinics. These institutions are responsible for purchasing, inventory management, clinical application, and patient care. Post-market activities, including device tracking, technical support, training for medical professionals, and adverse event reporting, form an essential part of the value chain, ensuring long-term product performance, patient safety, and regulatory compliance, thereby completing the cycle of device development and deployment.
Cardiovascular Devices Market Potential Customers
The primary potential customers and end-users of cardiovascular devices are diverse, spanning various healthcare institutions and professional groups responsible for diagnosing, treating, and managing cardiovascular diseases. Hospitals, particularly those with dedicated cardiology departments, interventional cardiology units, and cardiac surgery suites, represent the largest customer segment. These facilities require a comprehensive range of devices for both diagnostic procedures and complex therapeutic interventions, serving a broad spectrum of patients from acute emergencies to chronic disease management. Their purchasing decisions are often influenced by clinical efficacy, cost-effectiveness, technological advancement, and integration with existing infrastructure, alongside favorable reimbursement policies.
Ambulatory Surgical Centers (ASCs) and specialized cardiac clinics are also significant end-users, especially for less invasive procedures and diagnostic tests that do not require overnight hospitalization. These settings often prioritize devices that enable efficient patient throughput, rapid recovery, and reduced operational costs. Diagnostic centers and independent cardiology practices further contribute to the customer base, focusing on advanced imaging equipment, ECG systems, and other diagnostic tools essential for early detection and comprehensive patient evaluation. The growing trend towards outpatient care and minimally invasive procedures continues to expand the demand for cardiovascular devices within these specialized settings, driving product innovation tailored to their unique operational needs.
Furthermore, an emerging segment of potential customers includes home care settings, driven by the increasing adoption of remote patient monitoring devices for chronic cardiovascular conditions. Patients and their caregivers, under the guidance of healthcare professionals, utilize devices such as wearable ECG monitors, smart blood pressure cuffs, and pulse oximeters to manage their health outside traditional clinical environments. This shift towards decentralized care is supported by technological advancements in connectivity and data analytics, offering new market opportunities for manufacturers. Government healthcare agencies and research institutions also procure cardiovascular devices for public health programs, clinical trials, and medical research, playing a vital role in advancing cardiovascular medicine and informing future clinical guidelines.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | $72.5 Billion USD |
| Market Forecast in 2032 | $115.6 Billion USD |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Medtronic, Boston Scientific Corporation, Abbott Laboratories, Johnson & Johnson, Edwards Lifesciences Corporation, Siemens Healthineers AG, GE Healthcare (a GE Company), Koninklijke Philips N.V., Stryker Corporation, LivaNova PLC, B. Braun Melsungen AG, Getinge AB, Terumo Corporation, Cook Medical, MicroPort Scientific Corporation, Teleflex Incorporated, Zimmer Biomet Holdings, Inc., Shockwave Medical, Inc., Integra LifeSciences Holdings Corporation, Biosense Webster, Inc. (part of J&J) |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Cardiovascular Devices Market Key Technology Landscape
The Cardiovascular Devices Market is characterized by a rapidly evolving technology landscape, with continuous innovation aimed at improving efficacy, minimizing invasiveness, and enhancing patient safety and comfort. A prominent technological trend is the advancement in minimally invasive surgical techniques, which increasingly utilize catheter-based procedures, robotic assistance, and advanced imaging guidance. These techniques reduce recovery times, lower complication rates, and improve patient experience, making them highly preferred over traditional open surgeries. The development of smaller, more flexible catheters, steerable guidewires, and advanced visualization systems are integral to these procedural enhancements, allowing for precise navigation within the complex cardiovascular system.
Another critical area of technological innovation lies in imaging and diagnostic modalities. High-resolution echocardiography, advanced computed tomography (CT), and magnetic resonance imaging (MRI) systems provide unprecedented detail of cardiac structures and function, enabling earlier and more accurate diagnoses. The integration of artificial intelligence and machine learning algorithms with these imaging platforms is revolutionizing image analysis, automating measurements, detecting subtle abnormalities, and predicting disease progression with greater precision than ever before. Furthermore, remote patient monitoring technologies, including wearable ECG devices, smart blood pressure cuffs, and implantable loop recorders, are gaining significant traction. These devices leverage wireless connectivity and cloud-based platforms to provide continuous, real-time data, enabling proactive management of chronic conditions and reducing the need for frequent hospital visits.
Material science also plays a pivotal role, with ongoing research into advanced biomaterials that offer enhanced biocompatibility, durability, and reduced risk of rejection or infection for implantable devices like stents, heart valves, and pacemakers. Biodegradable stents and drug-eluting coatings are examples of innovations that improve long-term outcomes by supporting natural vessel healing and preventing restenosis. Moreover, the emergence of personalized medicine approaches, driven by genetic profiling and patient-specific data, is influencing device design and therapeutic strategies, moving towards highly tailored interventions. These technological advancements collectively contribute to a more effective, safer, and patient-centric approach to cardiovascular care, constantly pushing the boundaries of what is possible in the diagnosis and treatment of heart and vascular diseases.
Regional Highlights
- North America: Dominates the global market due to sophisticated healthcare infrastructure, high prevalence of CVDs, favorable reimbursement policies, high healthcare spending, and rapid adoption of advanced medical technologies. The U.S. is the largest contributor, with significant R&D investments and a strong presence of key market players.
- Europe: A mature market characterized by stringent regulatory standards, high adoption rates of innovative devices, and a focus on cost-effective healthcare solutions. Countries like Germany, France, and the UK are key contributors, driven by an aging population and government support for cardiovascular health initiatives.
- Asia Pacific (APAC): Expected to witness the highest growth rate during the forecast period. This growth is attributed to a large and rapidly aging population, increasing incidence of CVDs, improving healthcare infrastructure, rising disposable incomes, and growing medical tourism. China, India, and Japan are leading the regional market expansion.
- Latin America: An emerging market with growing investments in healthcare infrastructure, increasing awareness about cardiovascular diseases, and improving access to advanced medical treatments. Brazil and Mexico are key markets in this region, driven by economic development and a rising middle class.
- Middle East and Africa (MEA): Shows promising growth potential, supported by increasing healthcare expenditures, development of modern medical facilities, and a rising focus on managing lifestyle-related diseases. The UAE, Saudi Arabia, and South Africa are prominent markets, benefiting from government initiatives to upgrade healthcare services.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Cardiovascular Devices Market.- Medtronic
- Boston Scientific Corporation
- Abbott Laboratories
- Johnson & Johnson
- Edwards Lifesciences Corporation
- Siemens Healthineers AG
- GE Healthcare (a GE Company)
- Koninklijke Philips N.V.
- Stryker Corporation
- LivaNova PLC
- B. Braun Melsungen AG
- Getinge AB
- Terumo Corporation
- Cook Medical
- MicroPort Scientific Corporation
- Teleflex Incorporated
- Zimmer Biomet Holdings, Inc.
- Shockwave Medical, Inc.
- Integra LifeSciences Holdings Corporation
- Biosense Webster, Inc. (part of J&J)
Frequently Asked Questions
What are cardiovascular devices?
Cardiovascular devices are medical instruments, apparatus, or implants designed to diagnose, monitor, prevent, or treat conditions affecting the heart and blood vessels. They range from diagnostic tools like ECG machines and echocardiographs to therapeutic devices such as pacemakers, stents, and artificial heart valves, significantly improving patient outcomes for various cardiovascular diseases.
How is AI impacting the Cardiovascular Devices Market?
AI is profoundly impacting the market by enhancing diagnostic accuracy (e.g., AI-powered image analysis), enabling personalized treatment plans, improving surgical precision through robotics, and facilitating predictive analytics for early disease detection. It also aids in optimizing device performance and streamlining R&D, leading to more effective and patient-specific cardiovascular care solutions.
What are the primary drivers of growth for the Cardiovascular Devices Market?
The key drivers include the rising global prevalence of cardiovascular diseases, an aging population, continuous technological advancements in device design and functionality (e.g., minimally invasive techniques, AI integration), increasing healthcare expenditure, and supportive government initiatives promoting heart health and early disease detection.
What are the main challenges faced by the Cardiovascular Devices Market?
Major challenges include the high cost associated with advanced devices and procedures, which can limit accessibility, stringent and complex regulatory approval processes that delay market entry for innovations, the shortage of skilled healthcare professionals trained to use these advanced technologies, and concerns related to data privacy and cybersecurity for connected devices.
Which regions are leading in the adoption and innovation of cardiovascular devices?
North America currently leads the market due to its advanced healthcare infrastructure and high adoption rates of new technologies. Europe is also a significant market for innovation. The Asia Pacific region, particularly China and India, is projected to show the fastest growth due to a large patient pool, improving healthcare facilities, and increasing investments.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
