Central Nervous System Biomarkers Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427657 | Date : Oct, 2025 | Pages : 244 | Region : Global | Publisher : MRU
Central Nervous System Biomarkers Market Size
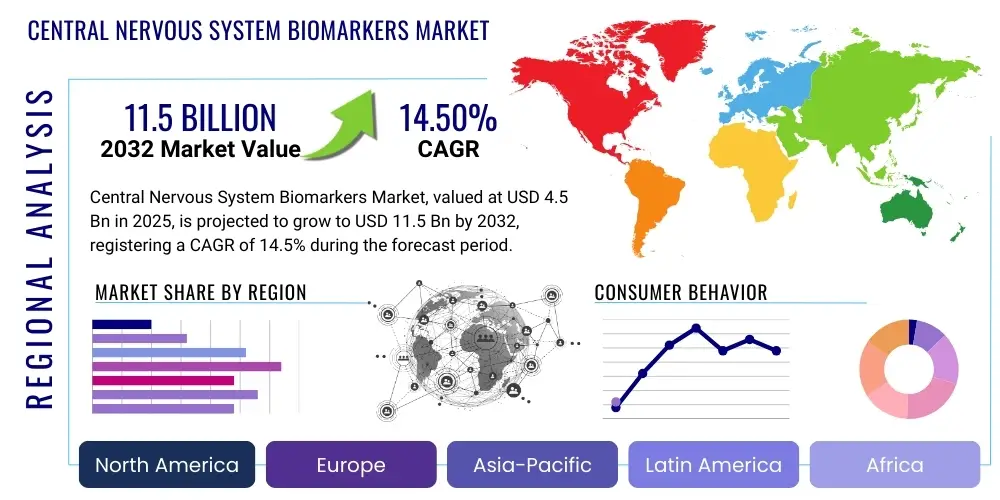
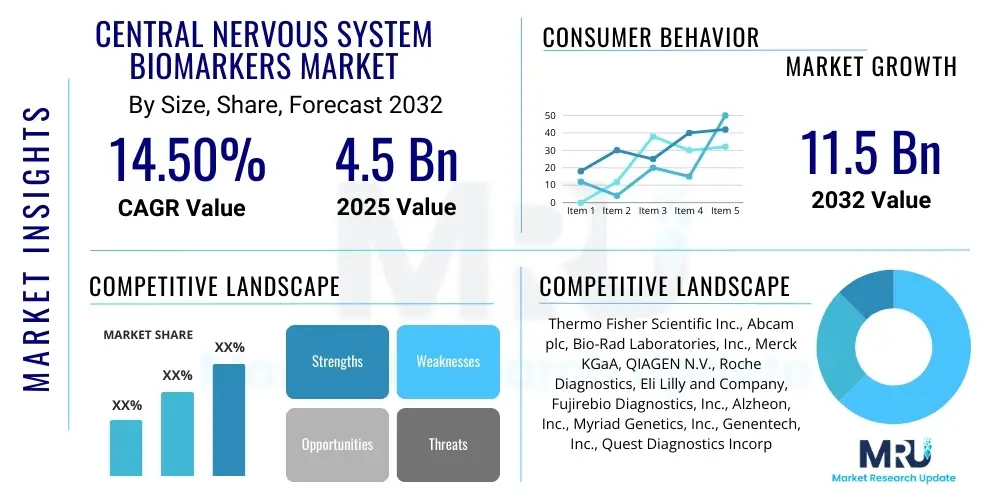
The Central Nervous System Biomarkers Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 14.5% between 2025 and 2032. The market is estimated at USD 4.5 billion in 2025 and is projected to reach USD 11.5 billion by the end of the forecast period in 2032.
Central Nervous System Biomarkers Market introduction
The Central Nervous System (CNS) Biomarkers Market encompasses a diverse range of measurable biological indicators that reflect the physiological or pathological state of the brain and spinal cord. These biomarkers are crucial for the early detection, diagnosis, prognosis, and monitoring of various neurological and psychiatric disorders, including Alzheimers disease, Parkinsons disease, multiple sclerosis, stroke, and depression. The market is driven by an increasing global prevalence of neurodegenerative diseases, significant advancements in biomarker discovery technologies, and a growing emphasis on personalized medicine approaches in neurology. These biological markers, which can be molecular, genetic, or imaging-based, provide objective and quantifiable insights into disease progression and treatment efficacy.
Product descriptions within this market span from protein-based biomarkers identifiable in cerebrospinal fluid (CSF) or blood, such as amyloid-beta and tau, to genetic markers indicative of disease susceptibility, and advanced imaging biomarkers derived from MRI, PET, or fMRI scans. Major applications include early disease diagnosis, differentiation of similar conditions, assessment of disease severity, prediction of patient response to therapies, and monitoring the impact of new drug candidates during clinical trials. The ability of these biomarkers to offer a more precise understanding of complex CNS disorders is revolutionizing neurological care and research, leading to more targeted interventions and improved patient outcomes.
The primary benefits of CNS biomarkers include enhanced diagnostic accuracy, enabling earlier and more precise interventions that can slow disease progression or alleviate symptoms. They significantly accelerate drug discovery and development processes by providing reliable endpoints for clinical trials, thereby reducing development costs and time. Furthermore, these biomarkers support the stratification of patient populations for clinical trials, ensuring that the right treatment reaches the right patient. Driving factors for market expansion include the aging global population, which correlates with a higher incidence of neurodegenerative conditions, increased funding for neurological research, and the emergence of advanced analytical techniques facilitating biomarker identification and validation.
Central Nervous System Biomarkers Market Executive Summary
The Central Nervous System Biomarkers Market is experiencing robust growth, primarily fueled by an escalating global burden of neurological disorders and significant technological breakthroughs in diagnostic and prognostic tools. Business trends indicate a strong focus on collaborative research between pharmaceutical companies, biotechnology firms, and academic institutions, aiming to accelerate the identification and validation of novel biomarkers. There is a discernible shift towards non-invasive or minimally invasive biomarker collection methods, such as blood-based assays, to improve patient compliance and expand accessibility, which is a key area of investment and innovation. Strategic alliances and mergers and acquisitions are also prominent, as companies seek to consolidate expertise, intellectual property, and market share in this rapidly evolving sector.
Regional trends reveal North America as a dominant market, largely due to extensive research funding, a high prevalence of CNS disorders, and the presence of leading pharmaceutical and biotech companies. Europe also holds a significant market share, driven by strong government support for healthcare research and well-established clinical trial infrastructure. The Asia Pacific region is anticipated to exhibit the highest growth rate, propelled by improving healthcare infrastructure, rising awareness of neurological conditions, and increasing investments in research and development activities in countries like China, India, and Japan. Latin America and the Middle East & Africa are emerging markets, with growing healthcare expenditures and increasing adoption of advanced diagnostic techniques contributing to their gradual expansion.
Segmentation trends highlight a strong demand for biomarkers related to Alzheimers disease and Parkinsons disease, given their high prevalence and unmet medical needs. Protein biomarkers, particularly those detectable in CSF and blood, currently lead the market, although genetic and imaging biomarkers are rapidly gaining traction due to advancements in sequencing and neuroimaging technologies. In terms of application, drug discovery and development continues to be a major segment, as pharmaceutical companies increasingly rely on biomarkers for patient selection, target validation, and efficacy monitoring. Diagnostic applications are also expanding, driven by the need for early and accurate diagnosis to enable timely therapeutic interventions and improve patient outcomes.
AI Impact Analysis on Central Nervous System Biomarkers Market
The integration of Artificial Intelligence (AI) and Machine Learning (ML) is poised to fundamentally transform the Central Nervous System Biomarkers Market by addressing challenges related to data complexity, pattern recognition, and predictive analytics. Users frequently inquire about AIs role in accelerating biomarker discovery, improving diagnostic accuracy, and personalizing treatment strategies. Key concerns often revolve around the validation of AI-derived insights, data privacy, and the ethical implications of autonomous diagnostic systems. Expectations are high regarding AIs ability to unlock insights from vast multi-modal datasets, including genomic, proteomic, clinical, and imaging data, leading to the identification of novel, more robust biomarker panels that are currently beyond the scope of traditional statistical methods. This analytical power is expected to enhance both the efficiency and effectiveness of biomarker-driven research and clinical practice.
AIs influence extends across the entire biomarker lifecycle, from initial discovery to clinical implementation. It can sift through enormous volumes of biological data, identifying subtle correlations and patterns that indicate disease presence or progression, significantly speeding up the identification of candidate biomarkers. In diagnostics, AI algorithms can analyze complex imaging data, such as MRI or PET scans, to detect early signs of neurodegeneration or abnormal activity patterns that might be missed by human observation, thereby improving the sensitivity and specificity of diagnostic tools. Furthermore, AI is critical in developing predictive models that can forecast disease trajectory, patient response to therapy, and even potential adverse drug reactions, making personalized neurology a more tangible reality.
The ability of AI to integrate diverse data sources—including clinical history, genetic profiles, biomarker levels, and lifestyle factors—allows for the creation of comprehensive patient profiles. This holistic view facilitates a more nuanced understanding of individual disease pathology, moving beyond a one-size-fits-all approach to treatment. While the promise of AI is immense, the market is also grappling with the need for standardized data formats, robust validation frameworks, and regulatory guidelines to ensure the reliability and clinical utility of AI-powered biomarker solutions. Addressing these challenges will be crucial for the widespread adoption and successful impact of AI in the CNS biomarkers landscape.
- Accelerated biomarker discovery through pattern recognition in large datasets.
- Enhanced diagnostic accuracy and early detection of CNS disorders using advanced algorithms.
- Improved predictive modeling for disease progression and treatment response.
- Integration of multi-modal data for comprehensive patient phenotyping.
- Personalization of neurological therapies based on AI-driven biomarker insights.
- Optimization of clinical trial design and patient selection processes.
- Development of AI-powered imaging biomarkers for subtle neurological changes.
- Automation of data analysis and interpretation, reducing human error and time.
DRO & Impact Forces Of Central Nervous System Biomarkers Market
The Central Nervous System Biomarkers Market is shaped by a powerful confluence of driving forces, significant restraints, and emerging opportunities, all interacting to create dynamic impact forces. A primary driver is the escalating global prevalence of neurodegenerative diseases such as Alzheimers, Parkinsons, and Multiple Sclerosis, creating an urgent demand for early and accurate diagnostic tools. Concurrently, substantial advancements in omics technologies—genomics, proteomics, and metabolomics—along with sophisticated imaging techniques, enable the identification and validation of novel biomarkers with greater precision and efficiency. Increased research funding from both governmental and private organizations, coupled with a growing focus on personalized medicine and precision neurology, further propels market expansion. These factors together are fostering an environment ripe for innovation and clinical translation of biomarker discoveries, profoundly influencing the entire R&D pipeline from discovery to patient care.
Despite the strong growth drivers, the market faces several significant restraints. The complex and heterogeneous nature of CNS disorders makes biomarker discovery and validation inherently challenging, often leading to high attrition rates for promising candidates. The lack of standardized protocols for sample collection, processing, and analysis across different research centers and diagnostic labs can lead to variability and reduce the reproducibility of results, hindering widespread clinical adoption. Regulatory hurdles are substantial, with stringent requirements for the approval of new diagnostic tests and therapies, which can prolong development timelines and increase costs. Furthermore, the high cost of advanced biomarker assays and imaging technologies can limit accessibility, particularly in developing regions, posing a barrier to market penetration and equitable healthcare provision.
Opportunities within the CNS Biomarkers Market are abundant and transformative. The development of non-invasive or minimally invasive biomarkers, especially blood-based assays, represents a significant growth avenue, promising easier patient access and improved compliance compared to traditional CSF-based methods. The integration of artificial intelligence and machine learning for analyzing complex, multi-modal biomarker data holds immense potential for uncovering previously undetectable patterns and improving diagnostic and prognostic accuracy. Moreover, the expanding pipeline of disease-modifying therapies for neurodegenerative diseases creates a crucial need for companion diagnostics to identify suitable patient populations and monitor treatment efficacy, thus driving demand for predictive and pharmacodynamic biomarkers. The increasing focus on precision medicine also provides a fertile ground for the development of highly specific biomarkers tailored to individual patient profiles, further propelling market innovation and clinical utility.
Segmentation Analysis
The Central Nervous System Biomarkers Market is segmented across various dimensions to provide a comprehensive understanding of its structure and dynamics. These segments often include distinctions by biomarker type, the specific neurological disease they target, their application within clinical and research settings, the end-user of these technologies, and the sample type utilized for analysis. This detailed segmentation enables market participants to identify niche opportunities, tailor product development, and refine strategic approaches based on specific market needs and technological capabilities. Each segment represents a critical component of the overall market, driven by unique technological advancements, regulatory considerations, and unmet medical needs.
Understanding these segmentations is crucial for navigating the market landscape, as it highlights areas of high growth and technological innovation. For instance, while protein-based biomarkers currently dominate, genetic and imaging biomarkers are rapidly gaining prominence due to advancements in sequencing and neuroimaging. Similarly, the demand for biomarkers in Alzheimers and Parkinsons disease remains high due to their increasing global prevalence, but there is also growing interest in markers for less common but equally debilitating conditions. The ongoing evolution across these segments reflects the dynamic nature of neurological research and the persistent drive to improve patient outcomes through more precise diagnostic and therapeutic tools.
- By Biomarker Type:
- Genetic Biomarkers
- Protein Biomarkers
- Metabolite Biomarkers
- Imaging Biomarkers (e.g., PET, MRI)
- Neurophysiological Biomarkers (e.g., EEG, ERP)
- By Disease:
- Alzheimers Disease
- Parkinsons Disease
- Multiple Sclerosis
- Stroke
- Epilepsy
- Depression
- Schizophrenia
- Traumatic Brain Injury (TBI)
- Others (e.g., ALS, Huntingtons Disease)
- By Application:
- Diagnostics
- Drug Discovery & Development
- Prognostics
- Personalized Medicine
- Disease Monitoring
- By End-User:
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic & Research Institutions
- Diagnostic Centers & Hospitals
- Forensic Laboratories
- By Sample Type:
- Cerebrospinal Fluid (CSF)
- Blood
- Urine
- Saliva
- Tissue
Central Nervous System Biomarkers Market Value Chain Analysis
The Central Nervous System Biomarkers Market value chain begins with upstream activities focused on basic research and biomarker discovery. This initial phase involves academic institutions, research organizations, and early-stage biotechnology companies identifying potential biological markers associated with CNS disorders. Key activities here include genomics, proteomics, metabolomics, and advanced imaging research, often involving high-throughput screening and bioinformatics analysis. Raw materials and specialized reagents, such as antibodies, enzymes, and chemical compounds, are sourced from various suppliers, forming the foundational inputs for biomarker development. The quality and availability of these upstream components significantly influence the efficiency and success of subsequent stages in the value chain, emphasizing the importance of robust R&D infrastructure and a reliable supply network.
Moving downstream, the value chain progresses through the validation, development, and commercialization of biomarker assays and diagnostic tools. This stage typically involves pharmaceutical and biotechnology companies, specialized diagnostic firms, and contract research organizations (CROs). Validation studies are critical to confirm the sensitivity, specificity, and reproducibility of candidate biomarkers, often requiring extensive clinical trials and regulatory compliance. Once validated, diagnostic kits, reagents, and imaging technologies are manufactured and packaged. Distribution channels play a vital role in connecting manufacturers with end-users, encompassing both direct sales forces for large pharmaceutical companies and indirect channels through distributors, wholesalers, and specialized medical device suppliers. This complex network ensures that products reach hospitals, diagnostic laboratories, research institutions, and ultimately, patients.
The distribution network for CNS biomarkers is bifurcated into direct and indirect channels. Direct channels involve manufacturers selling directly to large pharmaceutical companies, major academic research centers, or integrated healthcare systems, allowing for closer customer relationships and control over pricing and service. Indirect channels, conversely, utilize third-party distributors and wholesalers to reach a broader and more geographically dispersed customer base, especially smaller diagnostic labs and clinics. Both models have their advantages, with direct sales offering customization and specialized support, while indirect channels provide wider market penetration and logistical efficiency. The choice of distribution strategy often depends on the products complexity, the target markets size, and the manufacturers operational capabilities, all aimed at optimizing market reach and customer accessibility for these critical diagnostic and research tools.
Central Nervous System Biomarkers Market Potential Customers
The Central Nervous System Biomarkers Market caters to a diverse range of end-users and buyers, each with distinct needs and objectives. Pharmaceutical and biotechnology companies represent a significant customer segment, relying heavily on CNS biomarkers throughout their drug discovery and development pipelines. These companies utilize biomarkers for target identification and validation, patient stratification in clinical trials, monitoring drug efficacy and safety, and demonstrating treatment benefits for regulatory approval. Their demand is driven by the urgent need to develop effective therapies for neurological disorders and to reduce the high attrition rates associated with CNS drug development, making biomarkers indispensable tools for de-risking investments and accelerating market access for novel drugs.
Academic and research institutions constitute another crucial customer base. Researchers in universities and government-funded labs employ CNS biomarkers to unravel the complex pathophysiology of neurological diseases, identify new therapeutic targets, and conduct basic scientific investigations into brain health and disease. Their demand is often for research-use-only (RUO) products, including a wide array of reagents, assay kits, and analytical services that support fundamental scientific inquiry and preclinical studies. These institutions are at the forefront of biomarker discovery, often collaborating with industry partners to translate basic research findings into clinically actionable tools, thus fueling continuous innovation in the market.
Furthermore, diagnostic centers and hospitals are rapidly expanding as key end-users, particularly with the increasing availability of clinically validated CNS biomarker tests. These entities use biomarkers for early disease diagnosis, differential diagnosis, monitoring disease progression, and guiding treatment decisions for patients suffering from various neurological conditions. The demand from this segment is driven by the need for more accurate, timely, and less invasive diagnostic methods that can improve patient care outcomes. Contract Research Organizations (CROs) also form a vital customer group, as they provide specialized services to pharmaceutical and biotech companies, leveraging biomarkers in clinical trials, bioanalytical testing, and regulatory submissions, effectively bridging the gap between discovery and clinical application.
Central Nervous System Biomarkers Market Key Technology Landscape
The Central Nervous System Biomarkers Market is characterized by a rapidly evolving technology landscape, where continuous innovation drives the discovery, validation, and clinical application of novel markers. Immunoassays, such as Enzyme-Linked Immunosorbent Assays (ELISA) and Luminex-based multiplex assays, remain foundational technologies, widely used for the quantitative detection of protein biomarkers like amyloid-beta, tau, and neurofilament light chain (NfL) in biological fluids such as CSF and blood. These platforms offer high sensitivity and throughput, making them indispensable for both research and clinical diagnostics. Advancements in ultra-sensitive immunoassay technologies, such as Single Molecule Array (Simoa) by Quanterix, are significantly enhancing the detection limits, enabling the measurement of even minute quantities of biomarkers, particularly in less invasive samples like plasma.
Mass Spectrometry (MS) stands as a powerful and versatile technology in the CNS biomarker field, offering untargeted and targeted approaches for identifying and quantifying proteins, peptides, and metabolites. Techniques like Liquid Chromatography-Mass Spectrometry (LC-MS) and Gas Chromatography-Mass Spectrometry (GC-MS) are crucial for comprehensive proteomics and metabolomics studies, allowing researchers to discover new biomarker candidates and understand complex biochemical pathways involved in neurological diseases. MS provides high specificity and the ability to analyze multiple analytes simultaneously, making it invaluable for the discovery of novel biomarkers and the validation of existing ones, particularly in the context of personalized medicine where detailed molecular profiles are essential.
Beyond molecular assays, advanced neuroimaging techniques play a critical role as imaging biomarkers. Positron Emission Tomography (PET) scans, particularly those utilizing specific tracers for amyloid plaques or tau tangles, provide in vivo visualization of key pathological hallmarks of Alzheimers disease. Magnetic Resonance Imaging (MRI), including functional MRI (fMRI) and Diffusion Tensor Imaging (DTI), offers insights into brain structure, connectivity, and functional activity, aiding in the diagnosis and monitoring of conditions like multiple sclerosis and stroke. Furthermore, Next-Generation Sequencing (NGS) technologies are transforming the discovery of genetic biomarkers, identifying susceptibility genes and somatic mutations associated with various CNS disorders. The convergence of these diverse technological platforms, often augmented by bioinformatics and artificial intelligence, forms the bedrock of advancements in the CNS biomarkers market, offering unprecedented insights into neurological health and disease.
Regional Highlights
- North America: This region dominates the CNS biomarkers market, driven by substantial research funding from governmental and private organizations, a high prevalence of neurodegenerative disorders, and the presence of major pharmaceutical and biotechnology companies. The United States, in particular, benefits from advanced healthcare infrastructure, significant investments in R&D, and a robust regulatory framework that supports biomarker development and commercialization. Canada also contributes to market growth through its strong academic research sector and increasing healthcare expenditure.
- Europe: Europe represents a significant market share, characterized by a growing aging population, increasing incidence of neurological diseases, and strong government support for healthcare research initiatives. Countries such as Germany, the United Kingdom, France, and Switzerland are key contributors, leveraging well-established research institutions, clinical trial networks, and a focus on precision medicine. Regulatory bodies like the European Medicines Agency (EMA) facilitate the approval of innovative biomarker-based diagnostics and therapies.
- Asia Pacific: This region is projected to exhibit the highest CAGR during the forecast period, primarily due to improving healthcare infrastructure, rising awareness about neurological conditions, and increasing investments in healthcare R&D by both public and private sectors. Key countries like China, Japan, and India are emerging as major players, driven by a large patient pool, growing medical tourism, and a burgeoning biotechnology industry. Government initiatives to enhance healthcare access and technology adoption further fuel market expansion.
- Latin America: The market in Latin America is experiencing gradual growth, attributed to increasing healthcare expenditures, rising awareness of CNS disorders, and improving access to advanced diagnostic technologies. Countries such as Brazil and Mexico are leading the regional market, with efforts underway to strengthen research capabilities and adopt international standards for medical diagnostics.
- Middle East & Africa: This region is an emerging market for CNS biomarkers, characterized by a developing healthcare infrastructure and increasing investments in medical research. Growth is driven by a rising prevalence of neurological disorders and a growing demand for advanced diagnostic tools. Key growth areas include the UAE, Saudi Arabia, and South Africa, where governments are focusing on healthcare modernization and technological adoption.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Central Nervous System Biomarkers Market.- Thermo Fisher Scientific Inc.
- Abcam plc
- Bio-Rad Laboratories, Inc.
- Merck KGaA
- QIAGEN N.V.
- Roche Diagnostics (a division of F. Hoffmann-La Roche Ltd)
- Eli Lilly and Company
- Fujirebio Diagnostics, Inc.
- Alzheon, Inc.
- Myriad Genetics, Inc.
- Genentech, Inc. (a member of the Roche Group)
- Quest Diagnostics Incorporated
- Siemens Healthineers AG
- Euroimmun AG (a PerkinElmer Company)
- Quanterix Corporation
Frequently Asked Questions
What is a Central Nervous System biomarker?
A Central Nervous System (CNS) biomarker is a measurable indicator of a biological state or process within the brain or spinal cord. These markers can be molecules, genes, or imaging features that provide insights into neurological health, disease presence, progression, or response to treatment. They are crucial for the diagnosis, prognosis, and monitoring of various brain disorders.
How are CNS biomarkers used in drug development?
CNS biomarkers are extensively used in drug development to accelerate the process and improve success rates. They help in identifying and validating drug targets, stratifying patient populations for clinical trials, monitoring a drugs efficacy and safety, and demonstrating therapeutic effects. This reduces the time and cost associated with bringing new neurological treatments to market.
What are the key challenges in the CNS biomarkers market?
Key challenges include the complex heterogeneity of CNS disorders, which makes biomarker discovery difficult. Additionally, there is a lack of standardization in sample collection and analysis, high costs associated with advanced technologies, and stringent regulatory requirements for biomarker validation and approval, all of which can impede widespread clinical adoption.
Which technologies are crucial for CNS biomarker discovery?
Crucial technologies for CNS biomarker discovery and analysis include advanced immunoassay platforms (e.g., ELISA, Simoa), mass spectrometry (LC-MS, GC-MS) for proteomics and metabolomics, next-generation sequencing (NGS) for genetic analysis, and advanced neuroimaging techniques (PET, MRI) for visualizing brain changes in vivo.
What role does AI play in the CNS biomarkers market?
AI plays a transformative role by enhancing biomarker discovery through pattern recognition in large datasets, improving diagnostic accuracy via advanced algorithms, and enabling predictive modeling for disease progression and treatment response. AI also facilitates the integration of multi-modal data for personalized medicine, optimizing clinical trial design and accelerating insights from complex biological information.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager