Cough Assist Devices Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429684 | Date : Nov, 2025 | Pages : 258 | Region : Global | Publisher : MRU
Cough Assist Devices Market Size
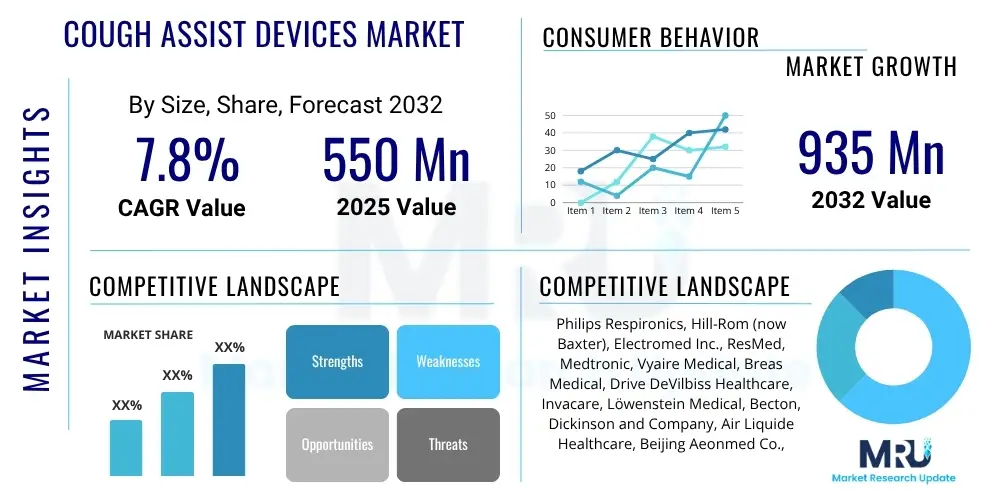
The Cough Assist Devices Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.8% between 2025 and 2032. The market is estimated at $550 million in 2025 and is projected to reach $935 million by the end of the forecast period in 2032.
Cough Assist Devices Market introduction
Cough assist devices, also known as mechanical insufflation-exsufflation (MIE) devices, are non-invasive medical technologies designed to help patients clear secretions from their lungs and airways when their natural cough reflex is impaired. These devices apply positive pressure to the airway to inflate the lungs, followed by a rapid shift to negative pressure to simulate a powerful cough. This action effectively dislodges and mobilizes mucus and other secretions, enabling patients to expel them more easily.
The primary applications of cough assist devices span a range of conditions that compromise respiratory muscle strength and coordination, including neuromuscular diseases like amyotrophic lateral sclerosis (ALS), muscular dystrophy, multiple sclerosis, and spinal cord injuries. They are also crucial for patients with chronic obstructive pulmonary disease (COPD), cystic fibrosis, and bronchiectasis. The significant benefits of these devices include reducing the incidence of respiratory infections, preventing atelectasis, improving oxygenation, and ultimately reducing hospitalizations and improving the overall quality of life for patients. They offer a safer, non-pharmacological alternative to more invasive airway clearance techniques.
Key driving factors for the cough assist devices market include the global rise in the prevalence of chronic respiratory diseases, an aging global population more susceptible to respiratory complications, and increasing awareness among healthcare professionals and patients about the efficacy and benefits of non-invasive airway clearance therapies. Technological advancements leading to more portable, user-friendly, and efficient devices, alongside growing adoption in home care settings, further propel market expansion. The shift towards home-based care models also contributes significantly, making these devices more accessible and integrated into daily patient management.
Cough Assist Devices Market Executive Summary
The Cough Assist Devices Market is poised for substantial growth, driven by escalating chronic respiratory disease prevalence and an aging demographic. Business trends indicate a strong emphasis on product innovation, focusing on enhanced portability, intuitive interfaces, and the integration of smart technologies for improved patient adherence and outcomes. Manufacturers are increasingly investing in research and development to produce quieter, more efficient, and feature-rich devices that cater to both clinical and home care environments, aiming to expand market reach and address diverse patient needs. The competitive landscape is characterized by strategic collaborations, mergers, and acquisitions as companies strive to consolidate their market position and leverage technological synergies.
Regional trends reveal North America and Europe as dominant markets due to well-established healthcare infrastructures, high healthcare expenditure, and significant awareness regarding advanced respiratory care solutions. These regions benefit from favorable reimbursement policies and a large patient pool suffering from neuromuscular and chronic respiratory conditions. However, the Asia Pacific region is emerging as the fastest-growing market, propelled by improving healthcare access, increasing disposable incomes, a large and aging population, and a rising incidence of respiratory ailments. Governments and healthcare organizations in APAC are progressively investing in modern medical technologies, expanding the adoption rates of cough assist devices across various clinical and home care settings.
Segment trends highlight a noticeable shift towards portable cough assist devices, favored for their convenience and ability to facilitate active lifestyles for patients, particularly in home care settings. This segment is experiencing accelerated growth as technological advancements make these devices lighter, more powerful, and battery-efficient. Furthermore, the application segment for neuromuscular disorders continues to represent a significant portion of the market, given the chronic nature of these conditions and the critical need for effective airway clearance. The increasing emphasis on home-based care is also shaping segment dynamics, driving demand for devices that are easy for patients and caregivers to operate without extensive medical training, thereby reducing the burden on institutional healthcare facilities and enhancing patient autonomy.
AI Impact Analysis on Cough Assist Devices Market
Common user questions regarding AI's impact on cough assist devices often revolve around enhanced diagnostic capabilities, personalized therapy, remote monitoring effectiveness, and predictive maintenance. Users are keen to understand how AI can make these devices smarter, more intuitive, and ultimately more effective in preventing respiratory complications. There is significant interest in AI's potential to analyze patient-specific data to optimize treatment parameters, provide early warnings for impending respiratory crises, and integrate seamlessly into broader telehealth ecosystems. The overarching expectation is that AI will elevate the standard of care by enabling more precise, proactive, and patient-centric airway clearance strategies, improving both device efficiency and long-term patient health outcomes while easing the burden on caregivers.
- Predictive analytics for early detection of respiratory exacerbations based on physiological data.
- Personalized therapy algorithms that adapt insufflation-exsufflation pressures and cycles to individual patient needs and real-time conditions.
- Enhanced remote patient monitoring through AI-driven data analysis, providing insights to healthcare providers and enabling timely interventions.
- Smart device diagnostics and maintenance alerts, predicting potential failures and optimizing device longevity.
- Voice command integration and intelligent user interfaces for improved ease of use and accessibility for patients with limited mobility.
- Automated coaching and feedback systems for patients and caregivers to ensure correct usage and adherence to therapy protocols.
- Data aggregation and analysis for research and development, leading to the design of more effective and targeted cough assist devices.
DRO & Impact Forces Of Cough Assist Devices Market
The Cough Assist Devices Market is significantly shaped by a combination of powerful drivers, inherent restraints, and emerging opportunities, all of which contribute to its overall impact forces. Key drivers include the escalating global prevalence of chronic respiratory diseases such as COPD, cystic fibrosis, and bronchiectasis, which necessitate effective airway clearance solutions. The rapidly expanding geriatric population, highly susceptible to respiratory infections and reduced cough effectiveness, further fuels demand for these devices. Moreover, continuous technological advancements leading to more portable, user-friendly, and efficient devices, coupled with increasing awareness among both medical professionals and patients about the benefits of non-invasive cough assistance, are propelling market growth. The growing emphasis on home healthcare settings also plays a crucial role, making devices more accessible and reducing healthcare facility burdens.
However, the market faces notable restraints that could impede its trajectory. The relatively high cost of advanced cough assist devices can be a significant barrier to adoption, particularly in developing economies or for patients without adequate insurance coverage. Limited reimbursement policies in certain regions and healthcare systems further restrict accessibility, making it challenging for some patients to afford necessary equipment. Additionally, the lack of adequately trained healthcare professionals or caregivers to properly operate and maintain these sophisticated devices, especially in home environments, poses a challenge to optimal patient outcomes. Potential side effects, though generally mild, such as discomfort, abdominal distension, or transient oxygen desaturation, can also deter some users, impacting patient compliance.
Despite these challenges, substantial opportunities exist for market expansion and innovation. Emerging markets across Asia Pacific, Latin America, and the Middle East and Africa present considerable growth potential as healthcare infrastructures improve and awareness of advanced respiratory therapies increases. The ongoing development of more compact, quieter, and smart-enabled devices with enhanced battery life offers avenues for product differentiation and wider adoption. Furthermore, the integration of cough assist devices with telehealth platforms and remote monitoring solutions presents a significant opportunity to improve patient management, track adherence, and provide virtual support, thereby enhancing patient care continuity. Strategic collaborations between manufacturers and home healthcare providers can also unlock new distribution channels and facilitate broader market penetration. The continuous evolution of regulatory frameworks to support the approval and use of these devices will also be a critical factor in fostering innovation and market access.
Segmentation Analysis
The Cough Assist Devices market is comprehensively segmented to provide a detailed understanding of its diverse components and the dynamics influencing various product types, applications, portability, and end-user groups. This segmentation allows for targeted strategic planning, identification of specific growth areas, and a clearer comprehension of patient needs and market demands. The categorization reflects the varied technological approaches, usage scenarios, and demographic characteristics that define the landscape of non-invasive airway clearance solutions.
- By Product Type
- Mechanical Insufflation-Exsufflation (MIE) Devices
- Percussive Nebulizers (as an adjunctive or related therapy)
- High-Frequency Chest Wall Oscillation (HFCWO) Devices (as an adjunctive or related therapy)
- By Portability
- Portable Devices
- Standalone/Tabletop Devices
- By Application
- Neuromuscular Disorders (e.g., ALS, Muscular Dystrophy, Multiple Sclerosis)
- Spinal Cord Injury
- Chronic Obstructive Pulmonary Disease (COPD)
- Cystic Fibrosis
- Bronchiectasis
- Others (e.g., post-surgical, pneumonia, asthma)
- By End User
- Hospitals
- Home Care Settings
- Specialty Clinics (e.g., Pulmonology, Neurology)
- Ambulatory Surgical Centers
Value Chain Analysis For Cough Assist Devices Market
The value chain for the Cough Assist Devices market encompasses a series of interconnected activities, beginning from raw material sourcing and extending through manufacturing, distribution, and ultimately to the end-users. Upstream activities involve the procurement of specialized components and raw materials crucial for device functionality, such as high-grade plastics for casings, precision motors for pressure generation, advanced sensors for monitoring, and sophisticated electronic components for control units and user interfaces. Suppliers in this segment play a critical role in ensuring the quality, reliability, and cost-effectiveness of these inputs, which directly impact the final product's performance and safety standards.
The midstream segment focuses on the manufacturing and assembly processes. This stage involves the meticulous assembly of various components, integration of complex software, rigorous quality control, and extensive testing to ensure devices meet stringent medical regulatory standards and performance specifications. Manufacturers invest heavily in research and development to innovate pressure control systems, enhance user ergonomics, and improve device portability and battery life. This stage is vital for creating a robust, reliable, and user-friendly product that adheres to global health and safety guidelines, setting the foundation for market acceptance and clinical efficacy.
Downstream activities center around the distribution and sales of cough assist devices. Distribution channels are varied, including direct sales forces engaging with large hospital networks and healthcare institutions, and indirect channels relying on medical device distributors, wholesalers, and increasingly, online medical supply retailers. These channels facilitate market reach, ensuring that devices are available to a broad spectrum of healthcare providers and individual patients. The distribution network also includes logistics, warehousing, and inventory management. Direct engagement allows manufacturers to maintain tighter control over sales and customer relationships, while indirect channels provide wider market penetration and leverage existing distribution infrastructures. Healthcare providers, including hospitals, specialty clinics, and home healthcare agencies, then interact directly with patients, prescribing and sometimes supplying the devices, often providing training and ongoing support for proper usage and maintenance. This comprehensive value chain ensures the efficient flow of products from innovation to the end-user, emphasizing quality, accessibility, and post-sales support.
Cough Assist Devices Market Potential Customers
The potential customers for cough assist devices are primarily individuals suffering from conditions that compromise their ability to cough effectively and clear airway secretions. This broad category includes patients diagnosed with various neuromuscular disorders, such as Amyotrophic Lateral Sclerosis (ALS), muscular dystrophy, multiple sclerosis, and spinal muscular atrophy, where respiratory muscle weakness is a common and progressive symptom. Patients with spinal cord injuries, particularly those affecting the cervical and thoracic regions, often experience significant impairment of their cough reflex due to paralysis of accessory respiratory muscles.
Beyond neurological conditions, a substantial patient base exists within the chronic respiratory disease community. This encompasses individuals with severe Chronic Obstructive Pulmonary Disease (COPD), cystic fibrosis, and bronchiectasis, who frequently contend with excessive mucus production and difficulty in expelling it, leading to recurrent infections and respiratory complications. These devices are critical for improving their quality of life, reducing hospitalizations, and preventing serious respiratory events. Moreover, patients recovering from major surgeries or those with critical illnesses who temporarily have a weakened cough reflex can also benefit from short-term cough assist therapy, making hospitals and rehabilitation centers key institutional buyers. Home healthcare providers and specialty clinics serving these patient populations are also significant end-users, facilitating the increasing trend toward home-based care for chronic conditions.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | $550 million |
| Market Forecast in 2032 | $935 million |
| Growth Rate | 7.8% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Philips Respironics, Hill-Rom (now Baxter), Electromed Inc., ResMed, Medtronic, Vyaire Medical, Breas Medical, Drive DeVilbiss Healthcare, Invacare, Löwenstein Medical, Becton, Dickinson and Company, Air Liquide Healthcare, Beijing Aeonmed Co., Ltd., DeVilbiss Healthcare LLC, PARI GmbH, Drägerwerk AG & Co. KGaA, Armstrong Medical Ltd., Seoil Pacific Co. Ltd., Amsino International Inc., CareFusion (now BD). |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Cough Assist Devices Market Key Technology Landscape
The technology landscape for cough assist devices is characterized by continuous innovation aimed at improving efficacy, user experience, and integration within broader healthcare ecosystems. Modern devices feature advanced pressure control systems that allow for precise regulation of insufflation and exsufflation pressures, optimizing therapy for individual patient needs while ensuring comfort and safety. These systems often include adjustable ramp times and customizable cycle durations, providing clinicians with greater flexibility in tailoring treatment protocols. The incorporation of real-time monitoring capabilities, such as integrated pulse oximetry, enables clinicians and caregivers to track oxygen saturation levels during therapy, ensuring immediate adjustments if needed and enhancing patient safety during the procedure. This data can also be logged and reviewed for long-term patient management and therapy optimization.
Significant technological advancements have focused on enhancing the user interface and connectivity of these devices. Touchscreen displays with intuitive menus are becoming standard, simplifying operation for both healthcare professionals and home users. Connectivity features, including Bluetooth and Wi-Fi, are increasingly integrated, allowing for seamless data transfer to electronic health records (EHRs) or dedicated cloud-based platforms for remote patient monitoring. This enables healthcare providers to remotely track therapy adherence, review usage patterns, and intervene proactively, aligning with the growing trend towards telehealth and decentralized care models. Improved battery technology has also led to more portable and lightweight devices with extended operating times, greatly enhancing mobility and convenience for patients who require therapy on the go.
Beyond core functionality, the market is seeing innovation in noise reduction technologies, making therapy less disruptive and more comfortable, particularly for overnight use. The development of more durable and hygienic components, including disposable filters and tubing, further addresses infection control concerns. Emerging technologies are also exploring the integration of artificial intelligence for personalized therapy adjustments based on real-time physiological feedback and predictive analytics to anticipate potential respiratory complications. This confluence of mechanical precision, smart connectivity, and user-centric design defines the cutting-edge of cough assist device technology, driving improved patient outcomes and expanded applications within diverse clinical and home care settings.
Regional Highlights
- North America: This region holds a dominant share in the cough assist devices market, driven by a high prevalence of chronic respiratory diseases, well-established healthcare infrastructure, and significant healthcare expenditure. Robust reimbursement policies, high awareness among healthcare professionals and patients, and the presence of leading market players contribute to its strong market position. The region also benefits from early adoption of advanced medical technologies and a focus on improving patient quality of life, particularly for those with neuromuscular disorders and chronic lung conditions.
- Europe: Europe represents a substantial market for cough assist devices, characterized by an aging population, a rising burden of respiratory illnesses, and sophisticated healthcare systems. Countries like Germany, France, and the UK are key contributors, benefiting from strong regulatory frameworks that support medical device innovation and widespread access to advanced medical treatments. Growing investment in home care and rehabilitation services also fuels market expansion across the European continent.
- Asia Pacific (APAC): The Asia Pacific region is projected to be the fastest-growing market, largely due to its immense population base, increasing healthcare spending, and improving access to medical facilities. Rising awareness of chronic respiratory conditions, coupled with a burgeoning geriatric demographic and improving economic conditions in countries such as China, India, and Japan, are driving the demand for effective airway clearance solutions. Government initiatives to enhance healthcare infrastructure and affordability also contribute significantly to market growth.
- Latin America: This region is an emerging market for cough assist devices, with growth primarily influenced by improving healthcare access and increasing medical awareness. Economic development and investments in healthcare infrastructure, particularly in countries like Brazil and Mexico, are gradually expanding the patient base capable of accessing and affording these devices. The rising prevalence of respiratory diseases in the region further contributes to steady market expansion.
- Middle East and Africa (MEA): The MEA market is still in its nascent stages but shows promising growth potential. Factors such as increasing healthcare investments, improving diagnostic capabilities, and a rising incidence of respiratory disorders are stimulating demand. While market penetration remains lower compared to developed regions, ongoing efforts to modernize healthcare systems and enhance patient care standards are expected to drive gradual but consistent growth in the forecast period.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Cough Assist Devices Market.- Philips Respironics
- Hill-Rom (now Baxter)
- Electromed Inc.
- ResMed
- Medtronic
- Vyaire Medical
- Breas Medical
- Drive DeVilbiss Healthcare
- Invacare
- Löwenstein Medical
- Becton, Dickinson and Company (BD)
- Air Liquide Healthcare
- Beijing Aeonmed Co., Ltd.
- DeVilbiss Healthcare LLC
- PARI GmbH
- Drägerwerk AG & Co. KGaA
- Armstrong Medical Ltd.
- Seoil Pacific Co. Ltd.
- Amsino International Inc.
- CareFusion (now BD)
Frequently Asked Questions
What are cough assist devices?
Cough assist devices, or mechanical insufflation-exsufflation (MIE) devices, are non-invasive medical machines designed to help patients with weak coughs clear lung secretions. They work by applying positive air pressure to inflate the lungs, followed by a rapid shift to negative pressure to simulate a natural cough, effectively mobilizing mucus.
Who typically needs a cough assist device?
Individuals with conditions causing impaired cough reflex, such as neuromuscular diseases (e.g., ALS, muscular dystrophy), spinal cord injury, or chronic respiratory conditions like severe COPD, cystic fibrosis, and bronchiectasis, are primary candidates for cough assist therapy.
How do cough assist devices improve respiratory health?
By simulating a strong, natural cough, these devices help to dislodge and remove mucus from the airways. This action reduces the risk of respiratory infections, prevents atelectasis (lung collapse), improves oxygenation, and ultimately contributes to better overall respiratory health and fewer hospitalizations.
Are cough assist devices covered by insurance?
Coverage for cough assist devices varies widely depending on the insurance provider, the patient's specific medical condition, and local reimbursement policies. In many regions, if deemed medically necessary by a physician, devices may be partially or fully covered, but patients should verify directly with their insurance plan.
What are the benefits of using a portable cough assist device at home?
Portable cough assist devices offer significant benefits for home use, including increased patient independence and mobility, greater convenience for daily therapy, reduced need for hospital visits, and improved adherence to treatment. They allow patients to maintain an active lifestyle while managing their respiratory health effectively.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
