Craniomaxillofacial Devices Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 428292 | Date : Oct, 2025 | Pages : 258 | Region : Global | Publisher : MRU
Craniomaxillofacial Devices Market Size
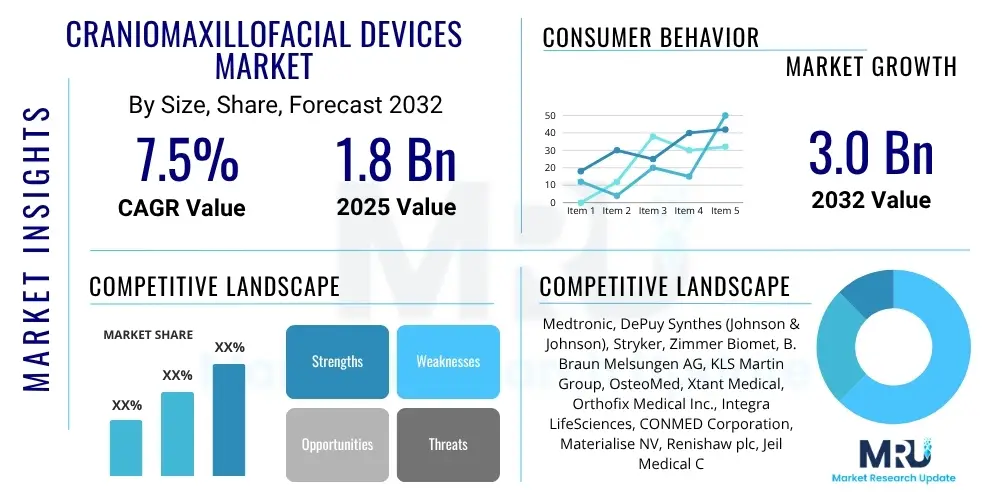
The Craniomaxillofacial Devices Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.5% between 2025 and 2032. The market is estimated at USD 1.8 billion in 2025 and is projected to reach USD 3.0 billion by the end of the forecast period in 2032.
Craniomaxillofacial Devices Market introduction
The Craniomaxillofacial (CMF) Devices Market encompasses a specialized range of medical implants, instruments, and technologies designed for surgical intervention in the head, face, and jaw regions. These devices are critical for treating a wide array of conditions, including traumatic injuries such as facial and skull fractures, congenital deformities like cleft lip and palate, orthognathic correction procedures for jaw misalignment, and reconstructive surgeries following tumor resections or other ablative procedures. Products typically include plates, screws, fixation systems, bone graft substitutes, distraction devices, and custom implants, often crafted from biocompatible materials such as titanium, PEEK (Polyetheretherketone), and various bioabsorbable polymers. The primary objective of these devices is to restore both the functional integrity and aesthetic appearance of the craniomaxillofacial structure, thereby significantly improving the patient's quality of life, mastication, speech, and overall self-esteem while minimizing long-term complications. The market's robust growth is significantly driven by factors such as the increasing global incidence of road accidents and sports-related injuries leading to facial trauma, a rising demand for aesthetic and reconstructive surgical procedures, an expanding aging population susceptible to age-related facial pathologies, and continuous advancements in surgical techniques and biomaterials, particularly in 3D printing for personalized solutions.
Craniomaxillofacial Devices Market Executive Summary
The Craniomaxillofacial Devices Market is experiencing dynamic shifts, characterized by several prominent business, regional, and segment trends. In terms of business trends, the market is witnessing a surge in mergers and acquisitions as major players consolidate their positions and expand their product portfolios, alongside a significant focus on research and development to introduce innovative materials and device designs, including bioresorbable implants and advanced fixation systems. The increasing adoption of personalized medicine, enabled by advanced imaging and 3D printing technologies for patient-specific implants and surgical guides, is transforming treatment approaches and enhancing surgical precision. Regionally, while mature markets in North America and Europe continue to lead in terms of technological adoption and healthcare expenditure, emerging economies in Asia Pacific and Latin America are poised for substantial growth. This growth is fueled by improving healthcare infrastructure, rising disposable incomes, increasing awareness regarding advanced medical treatments, and a higher incidence of trauma cases. Segment-wise, the trauma and fixation systems category remains dominant due to the high volume of facial and cranial fractures, but there is a notable expansion in the market for reconstructive and orthognathic surgery devices. The demand for advanced biomaterials such as PEEK and resorbable implants is growing steadily as they offer distinct advantages over traditional metal implants, including reduced stress shielding and artifact-free imaging. Furthermore, the integration of digital planning tools and navigation systems is enhancing the efficacy and safety of CMF procedures across all segments, driving demand for technologically advanced solutions.
AI Impact Analysis on Craniomaxillofacial Devices Market
The integration of Artificial Intelligence (AI) into the Craniomaxillofacial Devices Market is poised to revolutionize various aspects of patient care, from diagnostics and treatment planning to surgical execution and device design. Users frequently inquire about how AI can enhance the precision and predictability of CMF surgeries, reduce operative time, and improve patient outcomes. There is significant interest in AI's role in processing complex medical imaging data, such as CT and MRI scans, to generate highly accurate 3D models of anatomical structures, which are crucial for preoperative planning. This enables surgeons to meticulously plan complex procedures, anticipate potential challenges, and select optimal devices with unprecedented detail and confidence.
Furthermore, AI algorithms are being developed to analyze vast datasets of patient characteristics, surgical approaches, and outcomes, providing valuable insights for personalized treatment strategies. This includes predicting patient-specific risks, guiding the selection of appropriate implant materials and designs, and even automating certain aspects of custom implant design for 3D printing. The ability of AI to learn from a multitude of past cases allows for the identification of optimal surgical trajectories and the creation of patient-matched implants that perfectly conform to unique anatomical requirements, leading to improved functional and aesthetic results and a reduced need for revision surgeries.
The impact extends to operational efficiency and training, with AI-powered simulations offering realistic environments for surgeons to practice complex CMF procedures before operating on patients, thereby enhancing skill sets and reducing the learning curve for new techniques and devices. Despite these significant advancements, concerns around data privacy, regulatory approvals for AI-driven diagnostic and planning tools, and the initial investment required for AI infrastructure remain key areas of discussion among users. However, the overarching expectation is that AI will drive a paradigm shift towards more precise, personalized, and predictable outcomes in craniomaxillofacial surgery, fundamentally changing how devices are developed, selected, and utilized.
- Enhanced diagnostic accuracy through AI-powered image analysis for complex CMF conditions.
- Optimized preoperative planning and surgical simulation, leading to improved precision and reduced operative time.
- Personalized implant design and fabrication (via 3D printing) using AI algorithms for patient-specific anatomies.
- Predictive analytics to assess patient outcomes, identify potential complications, and guide treatment selection.
- Development of smart surgical tools and robotic-assisted systems with AI guidance for real-time adjustments.
- Streamlined workflow and increased efficiency in CMF device manufacturing and inventory management.
- Advanced training platforms utilizing AI for realistic surgical simulations and skill development.
DRO & Impact Forces Of Craniomaxillofacial Devices Market
The Craniomaxillofacial Devices Market is shaped by a confluence of robust drivers, significant restraints, and emerging opportunities, all influenced by various impact forces. Key drivers include the escalating global incidence of facial trauma resulting from road traffic accidents, sports injuries, and violence, which necessitates immediate surgical intervention. Furthermore, the growing demand for aesthetic and reconstructive procedures, driven by increasing awareness and advancements in surgical techniques, along with an expanding aging population prone to age-related degenerative conditions affecting the facial bones, significantly propel market expansion. Technological advancements in biomaterials, such as PEEK and bioresorbable polymers, coupled with the widespread adoption of 3D printing for custom implants and surgical guides, are also critical growth catalysts, offering superior patient-specific solutions and improved outcomes. However, the market faces notable restraints, including the high cost associated with advanced CMF devices and complex surgical procedures, which can limit accessibility in price-sensitive regions. Stringent regulatory approval processes for new devices, particularly those incorporating novel materials or AI components, pose significant challenges to market entry and product commercialization, extending development timelines and increasing costs. Moreover, a shortage of highly skilled oral and maxillofacial surgeons and neurosurgeons capable of performing intricate CMF procedures, especially in developing countries, acts as a bottleneck. Potential complications associated with surgical interventions, such as infection, implant failure, or nerve damage, also present a restraint as they influence patient and physician confidence. Opportunities for market players lie in tapping into underserved emerging markets with improving healthcare infrastructure, investing in the development of more affordable and efficient devices, exploring the integration of robotic-assisted surgery for enhanced precision, and expanding into niche areas like resorbable implants and advanced navigation systems. The market is also influenced by impact forces such such as the bargaining power of buyers, primarily hospitals and large healthcare systems, who demand cost-effective and high-quality solutions; the bargaining power of suppliers of specialized raw materials; the threat of new entrants with innovative technologies, though high R&D costs and regulatory hurdles limit this; the threat of substitute products, such as less invasive non-surgical treatments in certain aesthetic applications; and intense competitive rivalry among established global and regional players, driving continuous innovation and product differentiation.
Segmentation Analysis
The Craniomaxillofacial Devices Market is meticulously segmented across various dimensions, allowing for a granular understanding of its complex landscape and identifying distinct growth trajectories within each category. This segmentation typically covers aspects such as product type, application area, material composition, and end-user facilities, providing a comprehensive overview of how different technologies and solutions cater to specific medical needs and patient demographics. The diversity in product offerings, ranging from standard fixation systems to highly customized implants, reflects the varied requirements of CMF surgery. Similarly, the broad spectrum of applications underscores the critical role these devices play in addressing a wide array of conditions, from traumatic injuries to complex reconstructive challenges. Understanding these segmentations is vital for stakeholders to identify key growth areas, tailor product development strategies, and optimize market penetration efforts, ensuring that advancements in device technology align with clinical demands and patient outcomes.
- Product Type
- Plates and Screws
- Standard Plates and Screws
- Locking Plates and Screws
- Resorbable Plates and Screws
- Fixation Systems
- Internal Fixation Systems
- External Fixation Systems
- Bone Graft Substitutes
- Autografts
- Allografts
- Synthetics (e.g., Hydroxyapatite, Tricalcium Phosphate)
- Distraction Devices
- Internal Distractors
- External Distractors
- Craniofacial Implants
- Custom Implants
- Standard Implants
- Cranioplasty Plates and Meshes
- Dural Repair Products
- CMF Bone Cement and Augmentation Materials
- Other CMF Devices
- Plates and Screws
- Application
- Trauma
- Facial Fractures
- Skull Fractures
- Mandibular Fractures
- Midface Fractures
- Orthognathic Surgery (Jaw Correction)
- Neurosurgery (Cranial Fixation, Dural Closure)
- Reconstructive Surgery
- Congenital Deformities (e.g., Cleft Lip and Palate)
- Tumor Resection and Reconstruction
- Post-Traumatic Reconstruction
- Dental
- Dental Implants
- Maxillofacial Reconstruction for Dental Applications
- Aesthetic Surgery (e.g., Facial Contouring)
- Trauma
- Material
- Titanium and Titanium Alloys
- Polyetheretherketone (PEEK)
- Bioceramics (e.g., Hydroxyapatite, Calcium Phosphate)
- Bioabsorbable Materials (e.g., Polylactic Acid, Polyglycolic Acid)
- Stainless Steel
- Cobalt-Chrome Alloys
- End-User
- Hospitals
- Trauma Centers
- General Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics
- Oral & Maxillofacial Surgery Clinics
- Plastic Surgery Clinics
- Neurosurgery Clinics
- Academic and Research Institutes
- Hospitals
Value Chain Analysis For Craniomaxillofacial Devices Market
The value chain for the Craniomaxillofacial Devices Market is a complex and interconnected network that begins with upstream raw material suppliers and extends through manufacturing, distribution, and ultimately to end-users. The upstream segment involves the procurement of high-quality, biocompatible raw materials such as medical-grade titanium alloys, PEEK polymers, and bioabsorbable materials from specialized suppliers, who play a crucial role in ensuring the safety and efficacy of the final products. This is followed by intricate manufacturing processes that include precision machining, additive manufacturing (3D printing for custom implants), surface treatments, and sterilization, where device manufacturers convert these raw materials into finished CMF devices like plates, screws, fixation systems, and custom prosthetics. Downstream activities primarily involve the distribution and sales of these devices to various healthcare providers. Distribution channels are typically a hybrid model, encompassing both direct sales forces employed by major manufacturers to large hospitals and university medical centers, allowing for direct engagement, technical support, and training for surgeons. Alongside this, indirect distribution through a network of specialized third-party distributors and wholesalers is crucial for reaching smaller hospitals, ambulatory surgical centers, and specialty clinics, particularly in geographically dispersed regions. These distributors often provide logistical support, local inventory management, and regional market penetration, leveraging their established networks and relationships with healthcare professionals. The effectiveness of this value chain relies on stringent quality control at every stage, adherence to global regulatory standards, and continuous innovation in product design and manufacturing techniques to meet evolving clinical demands and patient needs.
Craniomaxillofacial Devices Market Potential Customers
The primary potential customers and end-users of Craniomaxillofacial Devices are diverse healthcare institutions and specialized medical practitioners who perform a wide range of surgical procedures involving the head, face, and jaw. Hospitals represent the largest segment of end-users, particularly major trauma centers that frequently manage severe facial and skull injuries, as well as general hospitals equipped with surgical departments dedicated to oral and maxillofacial surgery, plastic surgery, and neurosurgery. These facilities require a comprehensive array of CMF devices for both emergency interventions and planned reconstructive procedures. Ambulatory Surgical Centers (ASCs) are increasingly becoming significant customers, especially for less complex or elective CMF procedures, offering a cost-effective and convenient alternative to traditional inpatient hospital settings. Their growing prominence is driven by the shift towards outpatient care for suitable cases, demanding efficient and reliable CMF solutions. Specialty clinics, including dedicated oral and maxillofacial surgery clinics, plastic surgery clinics, and private neurosurgery practices, also constitute a vital customer base. These clinics cater to patients seeking specialized treatments for congenital deformities, aesthetic enhancements, or specific reconstructive needs, often requiring advanced and customized CMF implants. Additionally, academic and research institutes are potential customers, albeit on a smaller scale, as they utilize CMF devices for research, development of new surgical techniques, and training of future surgeons. The buying decisions within these customer segments are influenced by factors such as device efficacy, material biocompatibility, ease of use for surgeons, post-operative patient outcomes, cost-effectiveness, and the availability of comprehensive support services from manufacturers, including technical training and device customization options.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 1.8 billion |
| Market Forecast in 2032 | USD 3.0 billion |
| Growth Rate | 7.5% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Medtronic, DePuy Synthes (Johnson & Johnson), Stryker, Zimmer Biomet, B. Braun Melsungen AG, KLS Martin Group, OsteoMed, Xtant Medical, Orthofix Medical Inc., Integra LifeSciences, CONMED Corporation, Materialise NV, Renishaw plc, Jeil Medical Corporation, ShinWon Bionics, Aesculap, Inc., Cook Medical, W. L. Gore & Associates, Medartis AG, Titamed. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Craniomaxillofacial Devices Market Key Technology Landscape
The Craniomaxillofacial Devices Market is characterized by a rapidly evolving technological landscape, driven by the persistent pursuit of enhanced surgical precision, improved patient outcomes, and personalized treatment solutions. A cornerstone of this evolution is the widespread adoption of 3D printing (additive manufacturing) technologies, which enable the creation of highly customized, patient-specific implants and surgical guides from various biocompatible materials like titanium, PEEK, and bioabsorbable polymers. This allows for unparalleled anatomical fit and reduced operative time, significantly improving both functional and aesthetic results. Alongside 3D printing, advancements in medical imaging, particularly high-resolution CT and MRI scans, coupled with sophisticated image processing software, facilitate meticulous preoperative planning and virtual surgical simulation, ensuring greater predictability and safety during complex procedures. The integration of advanced navigation systems, which provide real-time intraoperative guidance and mapping, further augments surgical accuracy, particularly in complex anatomical regions of the cranium and face. Furthermore, the development of new and improved biomaterials, including enhanced PEEK variants, novel bioceramics, and next-generation bioabsorbable polymers with optimized degradation profiles, offers alternatives to traditional metal implants, minimizing issues like stress shielding and improving long-term tissue integration. The nascent but growing field of robotic-assisted surgery and augmented reality (AR)/virtual reality (VR) applications for surgical planning and execution also represent significant technological frontiers, promising to elevate precision, minimize invasiveness, and enhance training for CMF surgeons. These technologies collectively contribute to making CMF surgeries safer, more efficient, and tailored to individual patient needs, thereby driving innovation and growth across the market.
Regional Highlights
- North America: This region holds a significant market share, driven by a high incidence of facial trauma, advanced healthcare infrastructure, high healthcare expenditure, strong presence of key market players, and rapid adoption of innovative technologies like 3D printing and robotic surgery. Continuous R&D investments and favorable reimbursement policies also contribute to its dominance.
- Europe: Europe is a mature market with robust growth, primarily fueled by an aging population increasing the demand for reconstructive surgeries, well-established healthcare systems, and increasing awareness of advanced CMF solutions. Germany, France, and the UK are key contributors, characterized by technological advancements and high medical tourism for specialized procedures.
- Asia Pacific (APAC): Expected to be the fastest-growing region, APAC benefits from improving healthcare infrastructure, rising disposable incomes, increasing awareness about advanced medical treatments, and a large patient pool. Countries like China, India, and Japan are witnessing a surge in road accidents and sports injuries, alongside a growing demand for cosmetic and reconstructive surgeries.
- Latin America: This region shows promising growth potential due to increasing investments in healthcare infrastructure, improving economic conditions, and rising prevalence of facial injuries. Brazil and Mexico are leading markets, driven by a growing demand for both trauma care and aesthetic CMF procedures.
- Middle East and Africa (MEA): The MEA market is an emerging region with significant untapped potential. Growth is attributed to increasing government investments in healthcare, rising medical tourism, and a growing awareness of advanced surgical techniques. Countries like Saudi Arabia and UAE are making considerable efforts to modernize their healthcare sectors.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Craniomaxillofacial Devices Market.- Medtronic
- DePuy Synthes (Johnson & Johnson)
- Stryker
- Zimmer Biomet
- B. Braun Melsungen AG
- KLS Martin Group
- OsteoMed
- Xtant Medical
- Orthofix Medical Inc.
- Integra LifeSciences
- CONMED Corporation
- Materialise NV
- Renishaw plc
- Jeil Medical Corporation
- ShinWon Bionics
- Aesculap, Inc.
- Cook Medical
- W. L. Gore & Associates
- Medartis AG
- Titamed
Frequently Asked Questions
Analyze common user questions about the Craniomaxillofacial Devices market and generate a concise list of summarized FAQs reflecting key topics and concerns.What are Craniomaxillofacial Devices primarily used for?
Craniomaxillofacial (CMF) devices are specialized medical tools and implants used in surgical procedures on the skull, face, and jaw. Their primary applications include treating traumatic injuries such as facial and skull fractures, correcting congenital deformities, performing orthognathic surgeries for jaw alignment, and facilitating reconstructive procedures following tumor removal or other pathologies to restore both function and aesthetics.
What are the common materials used in Craniomaxillofacial Devices?
The most common materials utilized in CMF devices include medical-grade titanium and its alloys, known for their biocompatibility and strength. Other important materials are PEEK (Polyetheretherketone) for its radiolucency and mechanical properties similar to bone, various bioabsorbable polymers that dissolve over time, and bioceramics like hydroxyapatite for bone regeneration. Stainless steel and cobalt-chrome alloys are also used in specific applications.
How is 3D printing impacting the Craniomaxillofacial Devices market?
3D printing is significantly impacting the CMF market by enabling the creation of highly customized, patient-specific implants and surgical guides. This technology allows for precise anatomical fit, reduced surgical time, enhanced functional and aesthetic outcomes, and improved accuracy in complex reconstructive procedures. It represents a paradigm shift towards personalized medicine within CMF surgery, offering unparalleled precision in device design and application.
What are the main drivers for the growth of the Craniomaxillofacial Devices market?
Key growth drivers for the CMF devices market include the rising global incidence of facial trauma from accidents and injuries, increasing demand for both aesthetic and reconstructive surgical procedures, an expanding aging population susceptible to facial bone conditions, and continuous technological advancements in biomaterials and surgical techniques, particularly in areas like 3D printing and navigation systems. These factors collectively boost the demand for advanced CMF solutions.
Which geographical regions are showing significant growth in the Craniomaxillofacial Devices market?
While North America and Europe remain dominant with established markets and high healthcare spending, the Asia Pacific (APAC) region is projected to exhibit the fastest growth. This is driven by improving healthcare infrastructure, increasing disposable incomes, rising awareness of advanced medical treatments, and a growing patient pool in countries like China and India. Latin America and MEA also show promising growth due to healthcare investments and evolving medical practices.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
