Disposable Blood Pressure Cuffs Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 431303 | Date : Nov, 2025 | Pages : 255 | Region : Global | Publisher : MRU
Disposable Blood Pressure Cuffs Market Size
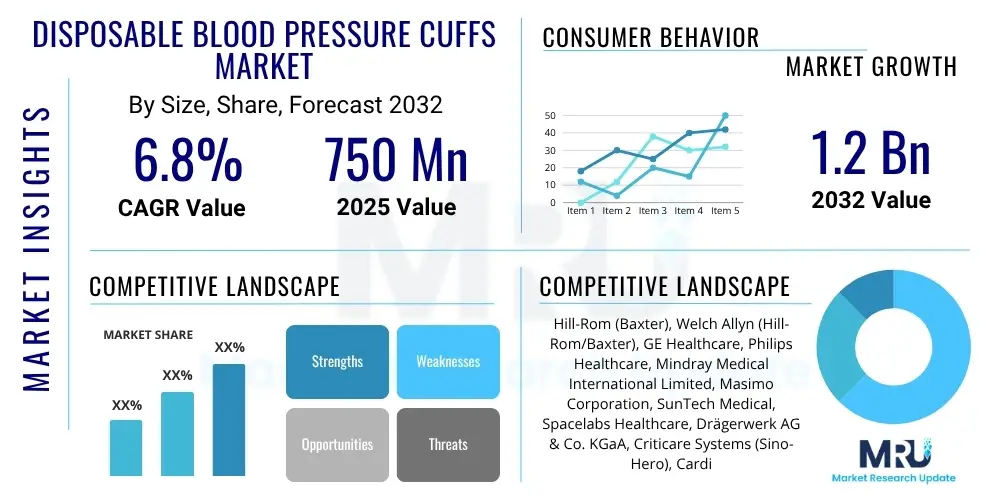
The Disposable Blood Pressure Cuffs Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2025 and 2032. The market is estimated at USD 750 million in 2025 and is projected to reach USD 1.2 billion by the end of the forecast period in 2032.
Disposable Blood Pressure Cuffs Market introduction
The Disposable Blood Pressure Cuffs Market encompasses a critical segment of medical devices designed for the accurate and hygienic measurement of arterial blood pressure in a variety of clinical and home settings. These single-patient use devices play a pivotal role in preventing cross-contamination and mitigating the incidence of healthcare-associated infections (HAIs), which are a significant concern in modern healthcare. The product range typically includes a comprehensive array of sizes, from neonatal and pediatric to adult and bariatric, ensuring appropriate fit and accurate readings across diverse patient populations. Cuffs are generally constructed from specialized materials such as non-woven fabrics, soft vinyl, or other biocompatible polymers, often featuring latex-free designs to prevent allergic reactions in sensitive patients, thereby enhancing patient safety and comfort during monitoring.
Major applications for disposable blood pressure cuffs are extensive, spanning across acute care hospitals, outpatient clinics, emergency medical services, long-term care facilities, and increasingly, home healthcare settings, where frequent and reliable blood pressure monitoring is indispensable. The paramount benefits derived from their use include significantly enhanced patient safety through rigorous infection control protocols, alongside substantial operational convenience for healthcare providers by eliminating the time-consuming and labor-intensive processes of cleaning, disinfection, and sterilization required for reusable cuffs. This efficiency contributes to improved clinical workflows and allows medical personnel to dedicate more time to direct patient care. Moreover, disposable cuffs offer consistent performance with each new application, removing variables associated with wear and tear, or incomplete sterilization processes often linked to reusable alternatives, thus ensuring the integrity of vital sign measurements.
The market's expansion is fundamentally driven by several powerful factors. The increasing global prevalence of chronic diseases such as hypertension, diabetes, and cardiovascular disorders necessitates continuous and accurate blood pressure monitoring, often over extended periods. Concurrently, the heightened global emphasis on infection prevention and control, particularly in the wake of public health crises, has solidified the preference for single-use medical devices. Furthermore, the burgeoning global geriatric population, which is inherently more susceptible to conditions requiring regular physiological assessments, constitutes a growing demographic driving demand. The rising adoption of telehealth and remote patient monitoring solutions also plays a crucial role, creating a sustained need for convenient and hygienic blood pressure measurement tools for at-home use, thereby expanding the market reach beyond traditional institutional settings.
Disposable Blood Pressure Cuffs Market Executive Summary
The Disposable Blood Pressure Cuffs Market is currently experiencing a period of robust expansion, propelled by an escalating global focus on patient safety and the imperative to mitigate healthcare-associated infections. This growth is further underpinned by the rising global incidence of chronic diseases that mandate regular blood pressure surveillance. Current business trends within the sector demonstrate a pronounced emphasis on technological advancements and product innovation, particularly in the development of materials that are not only latex-free and biocompatible but also environmentally sustainable. Manufacturers are increasingly exploring integrated "smart" features that enable seamless data connectivity and interoperability with digital health platforms, aiming to enhance the utility of these seemingly simple devices.
Strategic market activities include a visible trend towards consolidation among leading industry players, alongside the formation of strategic alliances and partnerships designed to optimize supply chains, broaden distribution networks, and penetrate new geographical territories. These collaborations are crucial for scaling operations and maintaining competitive advantage in a dynamically evolving healthcare landscape. Furthermore, the market is witnessing a surge in demand from the home care sector, driven by advancements in remote patient monitoring technologies and a societal shift towards managing chronic conditions outside of traditional hospital environments. This shift necessitates the availability of user-friendly, reliable, and hygienically sound disposable cuffs for personal use.
Geographically, while North America and Europe continue to represent mature markets with high rates of adoption, primarily attributed to their advanced healthcare infrastructures and stringent regulatory environments, the Asia Pacific region is rapidly emerging as a primary growth engine. This accelerated expansion in APAC is fueled by substantial increases in healthcare expenditure, significant improvements in medical facilities, and the sheer volume of its patient population, coupled with growing awareness regarding infection control. From a segmentation perspective, the adult cuff category retains its dominant market share due to the broad demographic it serves; however, the pediatric and neonatal segments are exhibiting accelerated growth, driven by enhanced global focus on maternal and child health outcomes and specialized clinical needs. This comprehensive overview underscores a dynamic market poised for continued innovation and global expansion.
AI Impact Analysis on Disposable Blood Pressure Cuffs Market
User questions concerning the influence of Artificial Intelligence on the Disposable Blood Pressure Cuffs Market frequently center on how AI can fundamentally transform the accuracy and utility of blood pressure monitoring. Users are keen to understand if AI can move beyond simple data logging to offer predictive health insights, facilitate seamless integration with existing electronic health records (EHRs), and significantly enhance remote patient management capabilities. Specific questions often delve into the potential for AI to personalize treatment protocols, identify subtle health deteriorations early, and manage complex data streams from continuous monitoring. Concerns also arise regarding data privacy, the ethical implications of AI-driven diagnostics, and the practical challenges of integrating advanced AI components into cost-effective, single-use medical devices.
The overarching themes emerging from these discussions indicate a strong expectation for AI to elevate blood pressure monitoring from a passive data collection exercise to an active, intelligent health management tool. There is a clear desire for AI to contribute to a more proactive and personalized approach to patient care, where insights derived from blood pressure data can be cross-referenced with other physiological markers and patient history to construct a holistic health profile. Users anticipate that AI will not only improve the precision of measurements but also provide actionable intelligence that supports clinical decision-making, streamlines operational efficiency, and ultimately contributes to superior patient outcomes. This shift is expected to extend the utility of disposable cuffs beyond their immediate function, embedding them within a sophisticated digital health ecosystem.
- Enhanced Data Analysis and Interpretation: AI algorithms can meticulously analyze vast quantities of blood pressure measurements, identifying intricate patterns, subtle trends, and anomalous readings with greater precision than traditional manual review. This leads to a deeper understanding of patient physiological states and responses to treatment.
- Predictive Diagnostics for Cardiovascular Events: Integrating AI allows for the development of predictive models capable of forecasting potential cardiovascular events, such as hypertensive crises or hypotensive episodes, by correlating real-time blood pressure data with historical patient information and other vital signs. This enables timely medical interventions.
- Seamless Electronic Health Record (EHR) Integration: AI-powered systems can automate the logging, categorization, and contextualization of blood pressure readings into electronic health records. This reduces the burden of manual data entry, minimizes human error, and ensures that comprehensive patient data is readily accessible for clinicians, enhancing continuity of care.
- Optimized Remote Patient Monitoring (RPM): For patients undergoing remote monitoring, AI is instrumental in sifting through continuous streams of data, prioritizing alerts based on clinical significance, and providing actionable insights to healthcare providers. This makes RPM more efficient, allowing for targeted and personalized telehealth interventions.
- Personalized Treatment and Medication Management: By analyzing an individual patient's unique blood pressure profile, responses to medication, and lifestyle factors, AI can contribute to the formulation of highly personalized treatment recommendations and adjustments to medication regimens, aiming for optimal therapeutic outcomes.
- Improved Clinical Workflow and Operational Efficiency: AI can streamline clinical workflows by automating routine tasks, such as generating reports or scheduling follow-ups based on blood pressure trends. For disposable cuffs specifically, AI could optimize inventory management by predicting demand based on patient census, admission rates, and seasonal health trends, ensuring availability while minimizing waste.
- Early Detection of Patient Deterioration: Continuous, AI-backed analysis of blood pressure, especially when combined with other vital signs, can facilitate the early detection of subtle changes indicating patient deterioration in critical care units, post-operative recovery, or in patients with chronic conditions. This allows for proactive medical responses, potentially preventing severe complications.
DRO & Impact Forces Of Disposable Blood Pressure Cuffs Market
The Disposable Blood Pressure Cuffs Market is significantly shaped by a powerful interplay of driving forces that propel its expansion, alongside inherent restraints that temper growth, and distinct opportunities that promise future avenues for development. The foremost driver is the critical imperative for stringent infection control within healthcare environments. With healthcare-associated infections (HAIs) posing a substantial threat to patient safety and increasing healthcare costs, disposable cuffs offer an indisputable advantage by ensuring a fresh, sterile device for each patient, thereby drastically minimizing cross-contamination risks. This aligns with global regulatory mandates and best practices for infection prevention. Moreover, the escalating global prevalence of chronic diseases such as hypertension, diabetes, and various cardiovascular disorders necessitates consistent and accurate blood pressure monitoring, often over extended periods, generating a steady demand for reliable monitoring solutions. Furthermore, the burgeoning global geriatric population, a demographic highly susceptible to chronic conditions and requiring frequent medical surveillance, contributes significantly to market growth.
Despite these robust drivers, the market navigates several notable restraints that pose challenges to widespread adoption. A primary concern is the relatively higher per-use cost of disposable cuffs compared to their reusable counterparts. For healthcare institutions, particularly those in resource-constrained settings or operating under tight budgetary limitations, this cost differential can be a significant barrier. Additionally, the environmental impact associated with the disposal of single-use medical plastics is a growing concern. As environmental sustainability gains prominence across industries, the volume of plastic waste generated by disposable cuffs raises questions among environmental advocates and healthcare organizations committed to eco-friendly practices, prompting a demand for more sustainable material innovations. Challenges also arise from the need for healthcare professionals to be adequately trained in the correct application and usage of various cuff types to ensure accurate readings, as improper technique can lead to diagnostic inaccuracies, regardless of the cuff's inherent quality.
Conversely, the market is rife with compelling opportunities that promise to catalyze future growth and innovation. The rapid expansion of telehealth and remote patient monitoring (RPM) initiatives, exacerbated by global health crises, has created a significant and sustained demand for convenient, reliable, and hygienically sound blood pressure measurement tools for home use. Disposable cuffs are perfectly positioned to meet this need, offering ease of use and reduced infection risk in unsupervised settings. The continuous development of "smart" cuffs, which may incorporate integrated sensors for enhanced data capture and seamless connectivity features to digital health platforms, represents another lucrative avenue for market expansion. These innovations promise to transform passive monitoring into proactive health management. Geographically, underserved emerging markets, particularly in Asia Pacific, Latin America, and parts of the Middle East and Africa, offer substantial untapped potential. As these regions experience improvements in healthcare infrastructure, increased healthcare expenditure, and rising health awareness, the adoption of advanced and hygienic medical devices like disposable blood pressure cuffs is expected to accelerate.
Beyond these specific drivers, restraints, and opportunities, broader "impact forces" continuously shape the market landscape. These include evolving regulatory frameworks for medical devices, which impose stringent quality and safety standards that manufacturers must meet. Technological advancements in materials science and sensor technology can rapidly introduce new product capabilities or production efficiencies. Economic shifts, such as inflation or changes in healthcare reimbursement policies, directly influence purchasing power and market accessibility. Lastly, public health crises and global pandemics profoundly underscore the importance of infection control and preparedness, invariably boosting the demand for single-use medical devices. These external forces necessitate agility and adaptability from market participants to remain competitive and responsive to changing global healthcare demands.
Segmentation Analysis
The Disposable Blood Pressure Cuffs Market undergoes extensive segmentation to meticulously categorize and analyze the diverse product offerings, specific patient requirements, and varied application environments within the broader healthcare industry. This detailed approach is crucial for dissecting market dynamics, identifying precise demand patterns, and understanding the specific growth drivers inherent in various sub-sectors. Such granular segmentation empowers manufacturers to strategically develop and tailor their product portfolios to meet distinct clinical needs, while also enabling healthcare providers to make informed decisions regarding device procurement that align with optimal patient care protocols, safety standards, and infection prevention strategies across their diverse operational settings.
Understanding the intricacies of each segment is vital for comprehensive market assessment. For instance, segmenting by type allows for differentiation based on anatomical application and patient size, recognizing that the unique physiological characteristics of neonates require vastly different cuff designs compared to those for adults or bariatric patients. Application-based segmentation highlights where demand is most concentrated, reflecting the operational requirements and patient throughput of different healthcare facilities. Material-based segmentation focuses on the construction components, which directly impact factors like comfort, durability, and biocompatibility. Finally, end-user segmentation directly correlates with patient demographics, underscoring the importance of patient-specific product development and marketing strategies.
- By Type
- Arm Cuffs: Standard cuffs designed for upper arm placement, widely used across all patient categories.
- Thigh Cuffs: Larger cuffs used for blood pressure measurement on the thigh, typically for bariatric patients or when arm access is restricted.
- Neonatal Cuffs: Specifically designed for newborns, these are very small, soft cuffs with gentle adhesion to avoid skin trauma.
- Pediatric Cuffs: Sized for infants and children, ensuring accurate readings without causing discomfort or arterial compression issues in smaller limbs.
- Other Specialty Cuffs: Includes conical cuffs for limb circumference variations, or cuffs designed for specific surgical or diagnostic procedures.
- By Application
- Hospitals: Intensive Care Units (ICUs), Emergency Departments, Operating Rooms, General Wards – high volume usage where infection control is paramount.
- Clinics: General physician offices, specialty clinics (e.g., cardiology, nephrology), outpatient diagnostic centers for routine check-ups and follow-ups.
- Ambulatory Surgical Centers (ASCs): Facilities performing same-day surgical procedures, requiring reliable monitoring for pre-op, intra-op, and post-op care.
- Home Care Settings: Increasingly important for remote patient monitoring, chronic disease management, and elderly care, emphasizing ease of use for non-clinical users.
- Emergency Medical Services (EMS): Used by paramedics and first responders in ambulances and at accident sites for immediate vital sign assessment.
- By Material
- Woven Fabric Cuffs: Often made from cotton or synthetic woven materials, providing durability and patient comfort.
- Non-woven Fabric Cuffs: Lightweight and cost-effective, typically made from polypropylene or polyester, preferred for high-volume, single-use scenarios.
- Vinyl/Plastic Cuffs: Easy to wipe clean (though disposable), sometimes used for their robust nature in certain applications.
- Other Materials: Includes advanced breathable materials, hypoallergenic coatings, or future eco-friendly and biodegradable options.
- By End User
- Adult Patients: The largest segment, covering the general adult population requiring routine or chronic blood pressure monitoring.
- Pediatric Patients: Encompasses infants, toddlers, and adolescents, demanding specialized sizes and gentle materials.
- Neonatal Patients: Newborns and premature infants, requiring the smallest, most sensitive cuffs to prevent injury and ensure accurate measurement on delicate skin.
Value Chain Analysis For Disposable Blood Pressure Cuffs Market
The value chain for the Disposable Blood Pressure Cuffs Market is a meticulously orchestrated sequence of activities that transforms raw materials into finished medical devices delivered to end-users, underscoring collaboration across multiple specialized entities. It commences with robust upstream activities focused on the sourcing, procurement, and initial processing of essential raw materials and components. This foundational stage involves a diverse set of suppliers providing specialized non-woven or woven fabrics, various grades of medical-grade plastics for bladders and connectors, biocompatible adhesives, and tubing. Key component manufacturers are also integral here, supplying precision-engineered inflation bladders, universal connectors designed for broad compatibility with monitoring equipment, and specialized tubing that ensures reliable pneumatic function. Strict quality control protocols are implemented at this stage to guarantee the integrity of materials and compliance with fundamental medical device standards, which are non-negotiable for patient safety.
Transitioning from raw materials, the midstream activities encompass the core manufacturing, assembly, and sterilization processes of the disposable blood pressure cuffs. This phase is predominantly managed by specialized medical device manufacturers who possess the requisite expertise in design, precision fabrication, and assembly of complex medical components. Adherence to Good Manufacturing Practices (GMP) and ISO standards is paramount, ensuring that each cuff meets stringent quality and performance criteria. Sterilization, often achieved through ethylene oxide (EtO) or radiation, is a critical step to ensure the devices are ready for clinical use and free from microbial contaminants. Subsequently, cuffs are meticulously packaged, often in individual sterile pouches, to maintain their integrity and cleanliness until the point of use. This stage involves significant investment in automated production lines and controlled environments to maximize efficiency and minimize contamination risks, reflecting the high-stakes nature of medical device manufacturing.
The downstream activities focus on the efficient distribution and sales of the finished disposable blood pressure cuffs to the vast and varied end-user base. This involves leveraging both direct and indirect sales channels to maximize market reach. Direct distribution typically targets large institutional buyers such as extensive hospital networks, integrated delivery systems, and influential group purchasing organizations (GPOs), allowing manufacturers to establish direct relationships and manage large volume contracts. Indirect channels are more expansive, utilizing a broad network of specialized medical distributors and wholesalers who then supply products to individual hospitals, smaller clinics, ambulatory surgical centers, long-term care facilities, and increasingly, retail pharmacies and online medical supply platforms catering to the burgeoning home care sector. Effective logistics, inventory management systems, and a responsive supply chain are critical at this stage to ensure timely delivery, product availability across diverse geographical markets, and responsiveness to fluctuating demand, ultimately connecting the product to the patient who benefits from its hygienic and accurate functionality.
Disposable Blood Pressure Cuffs Market Potential Customers
The Disposable Blood Pressure Cuffs Market serves a wide and varied spectrum of potential customers and end-users across the entire healthcare continuum, each driven by distinct needs for accurate, hygienic, and operationally efficient physiological monitoring. At the forefront are hospitals, which constitute the largest and most intensive customer segment. Within hospital environments, critical areas such as Intensive Care Units (ICUs), Emergency Departments (EDs), Operating Rooms (ORs), and post-anesthesia care units (PACUs) are significant consumers, given the constant patient admissions, high patient turnover, and the absolute necessity for rigorous infection control. General medical and surgical wards also utilize these cuffs extensively for routine vital sign assessments for all inpatients. The demand from hospitals is primarily driven by the need for universal infection prevention protocols and the sheer volume of patient encounters.
Beyond the acute care setting, clinics of various specialties represent a substantial customer base. This includes general practitioner offices, specialized clinics focusing on cardiology, nephrology, endocrinology, and diagnostic imaging centers, where routine blood pressure checks are integral to patient consultation and chronic disease management. These facilities prioritize the convenience and hygienic assurance offered by disposable cuffs, enhancing patient trust and streamlining clinic operations. Ambulatory surgical centers (ASCs) are another key segment, as they perform a high volume of outpatient procedures, requiring reliable blood pressure monitoring devices for pre-operative assessments, intra-operative surveillance, and post-operative recovery, all within environments focused on rapid patient turnover and infection risk mitigation.
Furthermore, the rapidly expanding home healthcare sector and individual consumers managing chronic conditions independently constitute a crucial and growing segment of potential customers. With the global shift towards telemedicine and remote patient monitoring, there is an increasing demand for user-friendly, reliable, and hygienically safe blood pressure cuffs that can be easily used by non-clinical individuals in their homes. This includes elderly individuals, patients with chronic hypertension, or those recovering from cardiovascular events who require regular self-monitoring. Emergency medical services (EMS) providers also represent a vital customer group, requiring durable and rapidly deployable disposable cuffs for pre-hospital assessments in often challenging and unpredictable environments. Ultimately, the market caters to any entity or individual involved in the regular, non-invasive measurement of blood pressure where infection control, convenience, and consistent accuracy are paramount considerations.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 750 million |
| Market Forecast in 2032 | USD 1.2 billion |
| Growth Rate | CAGR 6.8% |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Hill-Rom (Baxter), Welch Allyn (Hill-Rom/Baxter), GE Healthcare, Philips Healthcare, Mindray Medical International Limited, Masimo Corporation, SunTech Medical, Spacelabs Healthcare, Drägerwerk AG & Co. KGaA, Criticare Systems (Sino-Hero), Cardinal Health, B. Braun Melsungen AG, NIKKISO Co., Ltd., Omron Healthcare, American Diagnostic Corporation (ADC), Vyaire Medical, BD (Becton, Dickinson and Company), Infinium Medical, Riester (Halma plc), PDI Inc., Accutronics Ltd., Medline Industries, LP, Qardio, Inc., Schiller AG, BPL Medical Technologies Pvt Ltd. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Disposable Blood Pressure Cuffs Market Key Technology Landscape
The technology landscape for the Disposable Blood Pressure Cuffs Market, while seemingly straightforward in its core function, is undergoing continuous refinement and subtle innovation that significantly enhances device performance, patient safety, and integration within the broader digital healthcare ecosystem. While the fundamental principle of oscillometric measurement for non-invasive blood pressure remains central, key technological advancements are primarily observed in materials science, connectivity capabilities, and the seamless integration of data into patient management systems. There is a strong emphasis on developing advanced, biocompatible materials that are inherently latex-free to meticulously avoid allergic reactions and skin irritation in sensitive patient populations. Innovations extend to the development of non-woven fabrics that are not only softer and more comfortable for prolonged wear but also maintain superior durability and structural integrity throughout their single-patient use, ensuring consistent and accurate readings.
Furthermore, technological integration increasingly extends to the design and functionality of cuff connectors and tubing. This involves ensuring universal compatibility with a wide array of existing blood pressure monitors and patient monitoring systems, thereby reducing the complexities of equipment setup and minimizing the likelihood of connection-related errors or disconnections during critical patient monitoring. The standardization of these connection interfaces is a critical aspect, simplifying workflows for healthcare professionals and enhancing safety. While the disposable cuffs themselves are largely passive components in terms of active electronics, their design is being optimized to facilitate seamless interaction within "smart" healthcare environments. This includes the incorporation of elements that support efficient inventory management and patient tracking, such as embedded RFID tags or printable barcodes that allow for automated scanning and data capture.
Looking ahead, the evolving landscape anticipates further significant advancements driven by both clinical needs and environmental imperatives. Research and development are intensely focused on pioneering sustainable and biodegradable materials that can effectively reduce the ecological footprint of single-use medical devices without compromising performance or safety standards. Additionally, there is a growing trend towards incorporating more intuitive, error-reducing designs, such as clearer sizing guides or color-coded indicators, which simplify application for healthcare providers and even for patients in home care settings. The ultimate goal is to evolve disposable cuffs beyond their immediate measurement function, integrating them more deeply into comprehensive digital health solutions that capture, transmit, and analyze vital patient data efficiently and reliably, aligning with both environmental responsibility and the overarching objectives of clinical workflow optimization and enhanced patient outcomes in an increasingly digital healthcare landscape.
Regional Highlights
- North America: This region consistently dominates the Disposable Blood Pressure Cuffs Market, primarily attributable to its highly advanced healthcare infrastructure, high rates of adoption for disposable medical devices, and the presence of extremely stringent infection control policies and regulatory frameworks. The significant prevalence of chronic diseases across the United States and Canada, coupled with a high per capita healthcare expenditure, further solidifies its leading position. Continuous technological innovation and a strong focus on patient safety also contribute to its substantial market share.
- Europe: As a mature and well-regulated market, Europe demonstrates strong demand, driven by robust regulatory frameworks for medical devices, a widespread and increasing awareness of Healthcare-Associated Infections (HAIs), and a significant aging population which necessitates frequent and reliable blood pressure monitoring. Countries such as Germany, the United Kingdom, France, and Italy are pivotal revenue generators, often setting benchmarks for quality and patient care standards that favor disposable solutions.
- Asia Pacific (APAC): Positioned for the fastest growth, the APAC region is characterized by rapidly improving healthcare expenditure, substantial government and private investments in enhancing healthcare infrastructure, and an enormous patient population. Countries like China, India, and Japan are at the forefront of this expansion, fueled by rising health awareness, increasing access to modern medical facilities, and the growing adoption of Western healthcare practices. The increasing prevalence of lifestyle diseases also contributes significantly to market acceleration here.
- Latin America: This region exhibits steady and promising growth within the market, primarily propelled by expanding access to healthcare services, a burgeoning medical tourism sector, and a concerted shift towards preventative care and early disease management. Countries such as Brazil and Mexico are leading the market in this region, driven by governmental initiatives to upgrade healthcare facilities and increase the availability of essential medical supplies, including hygienic blood pressure monitoring solutions.
- Middle East and Africa (MEA): Emerging as a market with significant potential, the MEA region is experiencing growth attributed to increasing government healthcare initiatives, substantial investments in developing modern hospital infrastructure, and a growing emphasis on managing chronic illnesses. Countries like Saudi Arabia, the United Arab Emirates (UAE), and South Africa are key contributors, with ongoing efforts to improve public health standards and reduce infection rates in their evolving healthcare systems.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Disposable Blood Pressure Cuffs Market.- Hill-Rom (a Baxter International Inc. company)
- Welch Allyn (also part of Hill-Rom/Baxter International Inc.)
- GE Healthcare
- Philips Healthcare
- Mindray Medical International Limited
- Masimo Corporation
- SunTech Medical (a subsidiary of Halma plc)
- Spacelabs Healthcare (a division of OSI Systems)
- Drägerwerk AG & Co. KGaA
- Criticare Systems (part of Sino-Hero (Shenzhen) Bio-Medical Electronics Co., Ltd.)
- Cardinal Health Inc.
- B. Braun Melsungen AG
- NIKKISO Co., Ltd.
- Omron Healthcare, Inc.
- American Diagnostic Corporation (ADC)
- Vyaire Medical, Inc.
- BD (Becton, Dickinson and Company)
- Infinium Medical, Inc.
- Riester (a brand of Halma plc)
- PDI Inc.
- Accutronics Ltd.
- Medline Industries, LP
- Qardio, Inc.
- Schiller AG
- BPL Medical Technologies Pvt Ltd.
Frequently Asked Questions
Why are disposable blood pressure cuffs preferred over reusable ones in modern healthcare?
Disposable blood pressure cuffs are significantly preferred in modern healthcare settings primarily due to their superior infection control capabilities, drastically minimizing the risk of cross-contamination and the spread of healthcare-associated infections (HAIs), which are a major global concern. This practice aligns with stringent patient safety protocols and reduces the financial and clinical burden associated with HAIs. Furthermore, disposable cuffs offer significant operational efficiencies by eliminating the time, labor, and resources required for cleaning, disinfection, and sterilization processes associated with reusable cuffs, thereby optimizing clinical workflows and allowing healthcare professionals to focus more on direct patient care.
What are the key benefits of incorporating disposable blood pressure cuffs into clinical practice?
The key benefits of incorporating disposable BP cuffs into clinical practice are multifaceted. Foremost is the undeniable enhancement of patient safety through rigorous infection prevention. These cuffs also provide consistent and accurate readings with every use, as they are not subject to the wear and tear or potential damage from repeated cleaning cycles that can affect reusable cuffs. Moreover, they offer improved patient comfort due to their typically softer, latex-free materials, reducing the likelihood of skin irritation or allergic reactions. From an operational standpoint, they streamline clinical workflows by eliminating maintenance tasks, reducing labor costs, and simplifying inventory management, contributing to overall healthcare system efficiency.
How does the market for disposable BP cuffs vary across different global regions?
The market for disposable BP cuffs exhibits distinct regional variations driven by healthcare infrastructure, regulatory environments, and economic factors. North America and Europe currently lead in market share due to their advanced healthcare systems, stringent infection control policies, and high per capita healthcare spending. However, the Asia Pacific region is rapidly emerging as the fastest-growing market, propelled by increasing healthcare investments, a large and aging population, and rising awareness regarding hygiene and patient safety. Latin America and the Middle East & Africa also show promising growth as their healthcare sectors develop and modernize, adopting more advanced medical practices and devices.
What primary role does infection control play in driving the demand for disposable blood pressure cuffs?
Infection control serves as the paramount driver for the demand for disposable blood pressure cuffs. In any healthcare environment, minimizing the transmission of pathogens is critical. Disposable cuffs ensure that each patient receives a brand-new, uncontaminated device, thereby creating a crucial barrier against the spread of bacteria, viruses, and other microorganisms that might otherwise linger on reusable equipment. This adherence to strict infection prevention protocols is not only a clinical best practice but also a regulatory requirement in many regions, making disposable cuffs an indispensable tool for maintaining a sterile and safe patient environment and reducing the incidence of potentially life-threatening HAIs.
What significant innovations are anticipated in the technology of disposable blood pressure cuffs?
Significant innovations in disposable blood pressure cuff technology are expected to focus on several key areas. Firstly, there will be a strong emphasis on developing more sustainable and biodegradable materials to address growing environmental concerns associated with plastic waste, without compromising performance or safety. Secondly, advancements will likely include enhanced integration with digital health platforms, potentially through RFID or NFC tags for automated tracking and seamless data transfer to electronic health records and remote patient monitoring systems. Thirdly, improvements in design may focus on more intuitive application, better patient comfort, and potentially subtle sensor integrations for enhanced accuracy or additional physiological data capture, pushing towards a smarter, more environmentally conscious, and integrated monitoring solution.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
