Electronic Clinical Outcome Assessment Solution Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429360 | Date : Nov, 2025 | Pages : 246 | Region : Global | Publisher : MRU
Electronic Clinical Outcome Assessment Solution Market Size
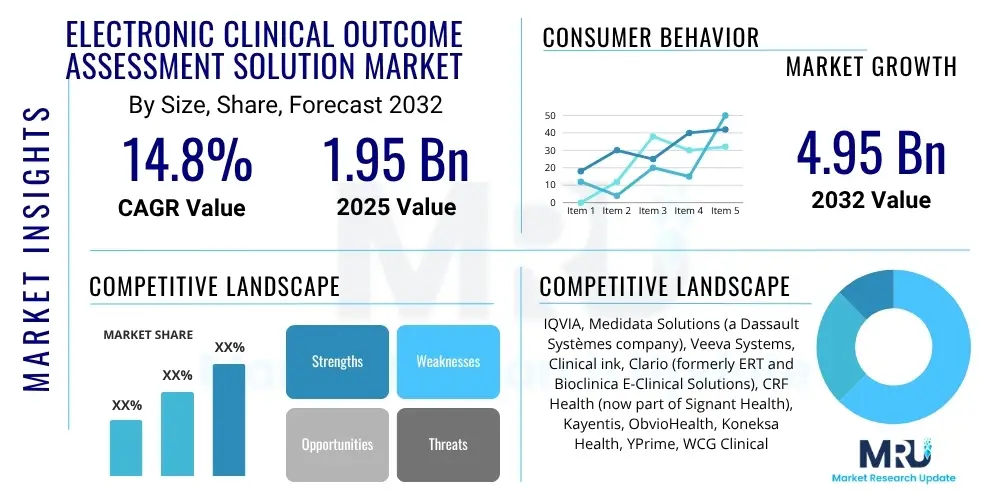
The Electronic Clinical Outcome Assessment Solution Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 14.8% between 2025 and 2032. The market is estimated at $1.95 billion in 2025 and is projected to reach $4.95 billion by the end of the forecast period in 2032.
Electronic Clinical Outcome Assessment Solution Market introduction
The Electronic Clinical Outcome Assessment (eCOA) Solution Market encompasses digital platforms and services designed to collect patient-reported outcomes (PROs), clinician-reported outcomes (ClinROs), observer-reported outcomes (ObsROs), and performance outcomes (PerfOs) in clinical trials and real-world studies. These solutions replace traditional paper-based methods, offering enhanced data accuracy, real-time data capture, and improved patient engagement throughout the research lifecycle. The primary objective of eCOA systems is to streamline data collection, reduce manual errors, and provide a more comprehensive and reliable dataset for evaluating the efficacy and safety of new medical interventions.
eCOA solutions are critically applied across a broad spectrum of clinical research, including phase I-IV clinical trials, observational studies, and patient registries in therapeutic areas such as oncology, central nervous system disorders, cardiovascular diseases, and rare diseases. They facilitate data collection directly from patients, investigators, or caregivers through various devices like smartphones, tablets, and web interfaces, thereby capturing subjective and objective measures that are vital for regulatory submissions and product development. The integration of eCOA into clinical workflows significantly enhances data integrity, reduces site burden, and accelerates the overall drug development timeline.
The core benefits of adopting eCOA solutions include superior data quality owing to built-in validation checks, improved patient compliance and engagement through user-friendly interfaces, and real-time access to data for more efficient trial monitoring and decision-making. Furthermore, eCOA systems support decentralized clinical trials by enabling remote data collection, enhancing patient access, and reducing travel burden, which is a significant driving factor in the current landscape. The increasing complexity of clinical trials, coupled with the rising demand for patient-centric data, continues to propel the demand for sophisticated eCOA platforms, making them indispensable tools for modern medical research.
Electronic Clinical Outcome Assessment Solution Market Executive Summary
The Electronic Clinical Outcome Assessment Solution Market is experiencing robust growth, primarily driven by the pharmaceutical and biotechnology industries' increasing embrace of digital transformation in clinical trials. Business trends indicate a strong shift towards more flexible, patient-centric trial designs, with eCOA solutions being a cornerstone of decentralized and hybrid trial models. Companies are investing heavily in platform integration capabilities, ensuring seamless data flow between eCOA systems, Electronic Data Capture (EDC), and other clinical trial management systems (CTMS). The competitive landscape is characterized by innovation in user interface design, advanced analytics features, and robust cybersecurity measures to protect sensitive patient data, all contributing to heightened market activity and strategic partnerships.
Regional trends highlight North America and Europe as the dominant markets for eCOA solutions, attributed to well-established pharmaceutical R&D infrastructures, stringent regulatory environments, and high adoption rates of advanced technologies in healthcare. However, the Asia Pacific region is emerging as the fastest-growing market, propelled by increasing investments in clinical research, a growing patient pool, and government initiatives promoting digitalization in healthcare. Latin America and the Middle East & Africa also show promising growth potential as global clinical trials expand into these regions, fostering the adoption of efficient data collection methods. This geographical expansion is further supported by the global imperative to conduct diverse and inclusive clinical studies.
Segment trends reveal that cloud-based eCOA solutions are gaining significant traction due to their scalability, accessibility, and cost-effectiveness compared to on-premise deployments. Patient-reported outcomes (PROs) remain a crucial segment, emphasizing the importance of direct patient input in assessing treatment efficacy and quality of life. The increasing complexity of clinical endpoints and the demand for real-time monitoring are boosting the demand for eCOA solutions across various therapeutic areas, particularly in oncology and chronic disease management. Service offerings, including consulting, implementation, and data management, are also expanding rapidly as companies seek comprehensive support for their eCOA deployments, underlining a preference for full-service providers.
AI Impact Analysis on Electronic Clinical Outcome Assessment Solution Market
The integration of Artificial intelligence (AI) into Electronic Clinical Outcome Assessment (eCOA) solutions is a transformative development, addressing common user questions about enhancing data quality, improving patient engagement, and accelerating insights from complex datasets. Users frequently inquire how AI can reduce errors, prevent data fraud, personalize assessments to individual patient needs, and provide predictive analytics for earlier intervention in clinical trials. They are also concerned about the ethical implications of AI, particularly regarding bias in algorithms, data privacy, and the transparency of AI-driven decision-making within eCOA platforms. The prevailing expectation is that AI will automate laborious tasks, uncover hidden patterns in patient data, and ultimately lead to more efficient and accurate clinical research outcomes, while ensuring regulatory compliance and maintaining the human element in patient care.
- AI enhances data quality through automated anomaly detection, identifying inconsistencies or potential fraud in real-time.
- Predictive analytics powered by AI can forecast patient adherence to protocols, helping to proactively manage engagement.
- Natural Language Processing (NLP) within eCOA can analyze free-text responses, extracting richer, nuanced insights from qualitative data.
- AI algorithms personalize assessment delivery, optimizing question sequences and timing based on individual patient responses and behavior.
- Machine learning models improve data synthesis and integration from disparate sources, offering a more holistic view of patient outcomes.
- AI assists in identifying early signals of adverse events or treatment non-response, enabling timely adjustments in trial design.
- Automated translation capabilities can broaden the global reach of eCOA by making assessments accessible in multiple languages without manual oversight.
- AI contributes to better cybersecurity by detecting unusual access patterns and potential breaches, safeguarding sensitive patient data.
DRO & Impact Forces Of Electronic Clinical Outcome Assessment Solution Market
The Electronic Clinical Outcome Assessment Solution Market is significantly propelled by several robust drivers, including the global shift towards patient-centric clinical trials, which necessitates direct patient input and real-time data capture to reflect treatment effectiveness accurately. The increasing complexity and cost of clinical development are also driving pharmaceutical and biotechnology companies to adopt eCOA to enhance efficiency, reduce manual errors, and accelerate regulatory submissions. Furthermore, the growing prevalence of chronic diseases and the subsequent rise in clinical research activities across various therapeutic areas demand more sophisticated and reliable data collection methodologies. The regulatory push for higher data quality and transparency from authorities like the FDA and EMA further reinforces the adoption of eCOA solutions, as they inherently provide a more robust audit trail and cleaner datasets.
However, the market also faces considerable restraints that could impede its growth. High initial investment costs associated with implementing eCOA platforms and integrating them with existing clinical trial infrastructure can be a barrier for smaller organizations or those with limited budgets. Concerns regarding data privacy and security, especially with the stringent requirements of regulations such as GDPR and HIPAA, present significant challenges for solution providers. There is also a notable lack of standardization across different eCOA platforms and a requirement for interoperability, making data exchange and aggregation complex. Additionally, a potential lack of digital literacy among some patient populations or investigators, particularly in developing regions, can hinder the widespread adoption and effective utilization of these advanced digital tools.
Despite these challenges, numerous opportunities are poised to fuel future market expansion. The increasing trend of decentralized and hybrid clinical trials, accelerated by recent global health events, creates a paramount need for robust remote data collection tools like eCOA. The integration of advanced technologies such as Artificial Intelligence (AI), Machine Learning (ML), and wearable devices into eCOA platforms promises to unlock new analytical capabilities, personalize patient experiences, and capture a broader range of physiological and behavioral data. Furthermore, the expansion into emerging markets, where clinical trial activities are rapidly increasing, offers significant untapped potential for eCOA solution providers. Strategic partnerships between technology firms, CROs, and pharmaceutical companies are also expected to drive innovation and broaden market reach, fostering more comprehensive and integrated solutions that address the evolving needs of clinical research.
Segmentation Analysis
The Electronic Clinical Outcome Assessment Solution Market is comprehensively segmented based on various critical attributes, providing a detailed understanding of its dynamics and growth trajectory. These segments encompass different aspects of the eCOA offerings, including the type of solution deployed, the mode of delivery, the components involved, the end-users leveraging these platforms, and the specific types of outcomes being assessed. This granular segmentation allows for a nuanced analysis of market trends, identifying areas of high growth and emerging opportunities across the diverse landscape of clinical research.
- By Type:
- Web-based eCOA Solutions: Platforms accessed via web browsers, offering flexibility and broad compatibility.
- Licensed Enterprise eCOA Solutions: On-premise or dedicated cloud deployments requiring direct licensing for organizational use.
- Cloud-based eCOA Solutions: Software-as-a-Service (SaaS) models hosted on cloud infrastructure, providing scalability and remote access.
- By Mode of Delivery:
- Web-hosted: Solutions delivered over the internet, accessible via web browsers or dedicated applications.
- On-premise: Software installed and maintained on the client's own servers and infrastructure.
- By Component:
- Software: The core eCOA platform, including modules for questionnaire design, data collection, and reporting.
- Services: Support services such as implementation, training, data management, technical support, and consulting.
- By End User:
- Pharmaceutical & Biopharmaceutical Companies: Major consumers for drug development clinical trials.
- Contract Research Organizations (CROs): Companies providing outsourced research services to pharma and biotech.
- Medical Device Companies: Utilizing eCOA for clinical evaluations of medical devices.
- Hospitals: Academic medical centers and hospitals conducting investigator-initiated trials.
- By Type of Outcome:
- Patient-Reported Outcomes (PROs): Data collected directly from patients about their health status and treatment impact.
- Clinician-Reported Outcomes (ClinROs): Assessments made by healthcare professionals based on their clinical observations.
- Observer-Reported Outcomes (ObsROs): Data reported by caregivers or family members, particularly for pediatric or cognitively impaired patients.
- Performance Outcomes (PerfOs): Objective measurements of a patient's functional ability based on specific tasks.
Value Chain Analysis For Electronic Clinical Outcome Assessment Solution Market
The value chain for the Electronic Clinical Outcome Assessment (eCOA) Solution Market begins with upstream activities focused on the foundational technologies and infrastructure providers. This segment includes companies specializing in software development tools, cloud computing infrastructure services, hardware manufacturers for mobile devices and tablets, and cybersecurity firms that provide essential data protection frameworks. These upstream players are critical in supplying the core technological components and secure environments upon which eCOA solutions are built. Their innovation in areas like robust data encryption, scalable cloud services, and advanced user interface design directly impacts the capabilities and reliability of the final eCOA product, forming the bedrock of the entire ecosystem.
In the midstream, the value chain involves the core activities of eCOA solution developers and service providers. This includes the design, development, and customization of eCOA platforms, encompassing questionnaire design tools, data capture modules, real-time analytics dashboards, and integration capabilities with other clinical trial systems like EDC and CTMS. These companies are responsible for ensuring regulatory compliance, user-friendliness, and the overall functionality of the eCOA system. They also provide crucial services such as implementation support, training for clinical trial staff and patients, data management, and ongoing technical support, which are essential for the successful deployment and operation of eCOA in diverse clinical research settings. The quality and comprehensiveness of these services are paramount to client satisfaction and trial integrity.
The downstream segment of the value chain focuses on the distribution channels and the end-users of eCOA solutions. Distribution primarily occurs through direct sales channels, where eCOA providers engage directly with pharmaceutical and biopharmaceutical companies, Contract Research Organizations (CROs), and medical device manufacturers. Partnerships with CROs are particularly significant, as CROs often recommend and integrate eCOA solutions into their comprehensive clinical trial services. Indirect channels may involve value-added resellers or system integrators who bundle eCOA platforms with other software solutions. The ultimate end-users, comprised of clinical trial sponsors, researchers, and patients, benefit from improved data collection efficiency, enhanced data quality, and a more streamlined clinical trial experience, which collectively drives the demand for eCOA solutions across the global healthcare research landscape.
Electronic Clinical Outcome Assessment Solution Market Potential Customers
The primary potential customers and end-users of Electronic Clinical Outcome Assessment (eCOA) solutions are organizations deeply involved in clinical research and drug development, seeking to enhance the efficiency, accuracy, and patient-centricity of their data collection processes. Pharmaceutical and biopharmaceutical companies represent the largest segment of potential customers, as they continually engage in extensive clinical trials for new drug approvals and post-market surveillance. These companies require robust eCOA platforms to meticulously collect patient-reported, clinician-reported, and other outcome data, ensuring compliance with stringent regulatory standards and generating high-quality evidence for their product pipelines. Their demand is driven by the need to accelerate drug development timelines, reduce operational costs, and improve the overall success rates of their clinical programs, making eCOA an indispensable tool in their research toolkit.
Contract Research Organizations (CROs) constitute another significant group of potential customers. CROs partner with pharmaceutical and biotechnology companies to conduct clinical trials on their behalf, offering specialized expertise and resources. As CROs manage a multitude of trials across various therapeutic areas, they require flexible, scalable, and highly integrated eCOA solutions that can be rapidly deployed and customized for diverse study protocols. The adoption of advanced eCOA platforms allows CROs to offer more competitive services, improve data quality, and streamline trial management for their clients, solidifying their position as crucial intermediaries in the clinical research ecosystem. Their operational model often necessitates solutions that can seamlessly integrate with existing EDC and CTMS, highlighting the importance of interoperability in eCOA offerings.
Beyond the core pharma and CRO sectors, the market extends to medical device companies, academic research institutions, and hospitals involved in clinical investigations. Medical device companies use eCOA to gather efficacy and safety data for new devices, often requiring tailored assessments for device-specific functionalities and patient experiences. Academic institutions and hospitals conducting investigator-initiated trials also benefit from eCOA by gaining access to cost-effective and efficient data collection methods, enabling them to conduct high-quality research and contribute to medical knowledge. This diverse customer base underscores the broad applicability and growing essentiality of eCOA solutions across the entire spectrum of healthcare innovation and research, driven by the universal need for reliable and patient-centric outcome data.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | $1.95 billion |
| Market Forecast in 2032 | $4.95 billion |
| Growth Rate | 14.8% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | IQVIA, Medidata Solutions (a Dassault Systèmes company), Veeva Systems, Clinical ink, Clario (formerly ERT and Bioclinica E-Clinical Solutions), CRF Health (now part of Signant Health), Kayentis, ObvioHealth, Koneksa Health, YPrime, WCG Clinical, Oracle Health Sciences, Bracket Global (now part of Clario), eClinical Solutions, OpenClinica, Florence Healthcare, Celerion, Castor, Curebase, uMotif. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Electronic Clinical Outcome Assessment Solution Market Key Technology Landscape
The Electronic Clinical Outcome Assessment (eCOA) Solution Market is underpinned by a dynamic and continuously evolving technology landscape, with several key innovations shaping its development and adoption. Cloud computing stands as a foundational technology, enabling scalable, secure, and globally accessible eCOA platforms. This infrastructure supports Software-as-a-Service (SaaS) models, allowing clinical trial sponsors and CROs to deploy solutions rapidly without significant upfront hardware investments, facilitating real-time data synchronization and remote access for geographically dispersed participants and researchers. Mobile technology, particularly smartphones and tablets, forms the primary interface for patient and clinician data input, requiring robust and intuitive application development that supports various operating systems and ensures a seamless user experience across diverse devices.
Advanced data analytics and Artificial Intelligence (AI) and Machine Learning (ML) are increasingly integral to eCOA solutions, moving beyond simple data collection to sophisticated insight generation. These technologies enable predictive modeling for patient adherence, anomaly detection for data quality assurance, and the identification of subtle patterns in vast datasets that might indicate treatment efficacy or adverse events. Natural Language Processing (NLP) is also being deployed to analyze free-text responses, extracting valuable qualitative data that complements structured questionnaire responses, thereby enriching the overall dataset. Furthermore, robust cybersecurity measures, including end-to-end encryption, multi-factor authentication, and compliance with data privacy regulations such as GDPR and HIPAA, are paramount to protecting sensitive patient health information and maintaining trust in digital data collection methods.
Interoperability standards and application programming interfaces (APIs) are critical for the seamless integration of eCOA platforms with other clinical trial management systems, such as Electronic Data Capture (EDC), Clinical Trial Management Systems (CTMS), and Electronic Health Records (EHR). This interconnectedness ensures a holistic view of patient data and streamlines workflows, eliminating data silos and reducing the burden of manual data transfer. The emergence of wearable technology and remote monitoring devices further expands the capabilities of eCOA, allowing for continuous and passive collection of physiological data, which provides objective and real-world evidence to complement traditional subjective outcomes. Blockchain technology is also being explored for its potential to enhance data integrity, auditability, and security within the eCOA ecosystem, offering a decentralized and immutable record of data transactions and patient consent processes.
Regional Highlights
- North America: This region holds a dominant share in the eCOA solution market, driven by a robust pharmaceutical and biotechnology industry, substantial R&D investments, and a proactive approach to adopting advanced clinical trial technologies. The presence of major eCOA vendors and stringent regulatory frameworks from agencies like the FDA further encourage the use of sophisticated digital data collection tools. High digital literacy rates and a strong emphasis on patient-centric research also contribute to the region's market leadership.
- Europe: The European market is a significant contributor to the eCOA landscape, characterized by a well-developed healthcare infrastructure, a large number of clinical trials, and favorable government initiatives promoting digitalization in clinical research. Countries like the UK, Germany, and France are at the forefront of eCOA adoption, driven by the need for efficient data management and compliance with regulations such as GDPR, which emphasizes data privacy and security in clinical studies.
- Asia Pacific (APAC): Expected to be the fastest-growing region, APAC offers immense opportunities for the eCOA market. This growth is fueled by increasing investments in healthcare R&D, a vast patient population, rising prevalence of chronic diseases, and a growing number of clinical trials being conducted in countries like China, India, Japan, and South Korea. Improving digital infrastructure and government support for advanced healthcare technologies are key catalysts.
- Latin America: The eCOA market in Latin America is in an emerging phase but shows promising growth. Factors such as increasing healthcare expenditure, growing awareness of the benefits of digital solutions in clinical research, and a rising number of international clinical trials expanding into the region are driving adoption. Brazil, Mexico, and Argentina are key markets with evolving regulatory landscapes and improving technological infrastructure.
- Middle East and Africa (MEA): This region is also an emerging market for eCOA solutions, characterized by nascent but growing clinical research activities and increasing government focus on modernizing healthcare. While adoption rates are currently lower compared to other regions, opportunities arise from expanding healthcare investments, the rise of chronic diseases, and the potential for new clinical trial sites, particularly in countries like Saudi Arabia, UAE, and South Africa.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Electronic Clinical Outcome Assessment Solution Market.- IQVIA
- Medidata Solutions (a Dassault Systèmes company)
- Veeva Systems
- Clinical ink
- Clario (formerly ERT and Bioclinica E-Clinical Solutions)
- CRF Health (now part of Signant Health)
- Kayentis
- ObvioHealth
- Koneksa Health
- YPrime
- WCG Clinical
- Oracle Health Sciences
- Bracket Global (now part of Clario)
- eClinical Solutions
- OpenClinica
- Florence Healthcare
- Celerion
- Castor
- Curebase
- uMotif
Frequently Asked Questions
What is an Electronic Clinical Outcome Assessment (eCOA) solution?
An eCOA solution is a digital platform used in clinical research to electronically collect patient-reported outcomes (PROs), clinician-reported outcomes (ClinROs), observer-reported outcomes (ObsROs), and performance outcomes (PerfOs), replacing traditional paper-based methods for enhanced data quality and efficiency.
Why are eCOA solutions important for clinical trials?
eCOA solutions are crucial for clinical trials because they improve data accuracy and completeness, provide real-time access to outcome data, enhance patient engagement and compliance, and support the shift towards more patient-centric and decentralized trial designs, ultimately accelerating drug development.
What are the key benefits of using eCOA over paper-based methods?
Key benefits include superior data quality with built-in validation, reduced data entry errors and missing data, faster data capture and real-time monitoring, improved patient adherence through user-friendly interfaces, and reduced logistical burden for both patients and clinical sites.
How does AI impact the Electronic Clinical Outcome Assessment market?
AI impacts the eCOA market by enhancing data quality through anomaly detection, personalizing patient assessments, providing predictive analytics for patient adherence and adverse events, and improving data synthesis from various sources, leading to more efficient and insightful clinical research.
Which regions are leading the adoption of eCOA solutions globally?
North America and Europe are currently leading the global adoption of eCOA solutions due to established pharmaceutical industries, significant R&D investments, and stringent regulatory environments. The Asia Pacific region is also experiencing rapid growth and is expected to become a major market player.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
