External Ventricular Drain Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429063 | Date : Oct, 2025 | Pages : 243 | Region : Global | Publisher : MRU
External Ventricular Drain Market Size
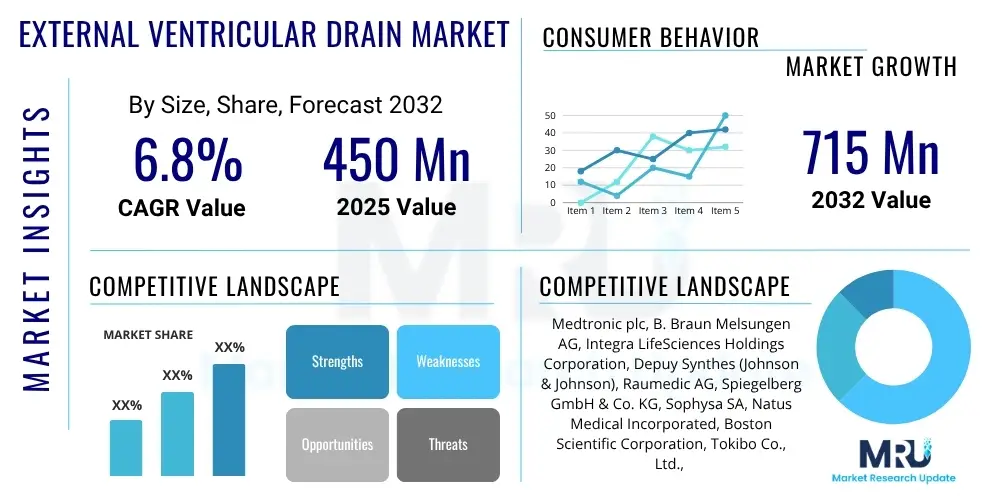
The External Ventricular Drain Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2025 and 2032. The market is estimated at USD 450 million in 2025 and is projected to reach USD 715 million by the end of the forecast period in 2032.
External Ventricular Drain Market introduction
The External Ventricular Drain (EVD) Market encompasses the medical devices and associated equipment used to drain cerebrospinal fluid (CSF) from the ventricles of the brain, thereby managing intracranial pressure (ICP) and providing diagnostic samples. EVDs are critical in neurocritical care for patients suffering from conditions such as hydrocephalus, traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), and intracerebral hemorrhage (ICH). These systems provide a direct means of reducing dangerously high ICP, which can prevent secondary brain injury and improve patient outcomes.
A typical EVD system consists of a catheter inserted into a cerebral ventricle, connected to an external drainage system that includes a collection bag, tubing, and a pressure transducer for continuous ICP monitoring. The product design emphasizes patient safety, ease of insertion, and reliable performance in demanding clinical environments. Major applications involve the acute management of various neurological emergencies where CSF accumulation or elevated ICP poses a life-threatening risk. The ability to precisely control CSF drainage and monitor ICP in real-time is a significant benefit, guiding clinical decisions and facilitating timely interventions.
The market's expansion is fundamentally driven by the escalating global incidence of neurological disorders, an aging demographic more susceptible to these conditions, and continuous advancements in neurosurgical techniques that necessitate sophisticated ICP management solutions. Increased awareness regarding timely diagnosis and intervention for brain injuries and pathologies also contributes significantly to the adoption of EVDs. The benefits of improved patient care and reduced mortality associated with effective ICP management further bolster the demand for these crucial medical devices.
External Ventricular Drain Market Executive Summary
The External Ventricular Drain Market is experiencing dynamic shifts, driven by persistent advancements in neurocritical care and a rising global burden of neurological conditions. Business trends indicate a strong focus on research and development, particularly towards creating EVD systems with enhanced safety features, such as antimicrobial coatings and integrated smart monitoring capabilities. The competitive landscape is characterized by both established medical device giants and specialized niche players, all striving to innovate and capture market share through product differentiation and strategic collaborations. Furthermore, there is a noticeable trend towards the adoption of single-use, disposable EVD kits to minimize infection risks and streamline hospital procedures, influencing procurement and supply chain strategies.
Regionally, mature healthcare markets in North America and Europe continue to be significant revenue contributors, benefiting from advanced healthcare infrastructure, high incidence of neurotrauma, and robust reimbursement policies. However, the Asia Pacific region is rapidly emerging as a high-growth market, propelled by increasing healthcare expenditure, improving access to advanced medical treatments, and a growing patient population. Latin America and the Middle East and Africa regions, while smaller in market size, present considerable opportunities for growth due to developing healthcare systems and a rising demand for critical care solutions. These regional disparities necessitate tailored market entry and expansion strategies for manufacturers.
Segmentation trends reveal that disposable EVDs dominate the market due to their superior infection control benefits and ease of use, gradually phasing out reusable components. In terms of application, hydrocephalus and traumatic brain injury collectively account for a substantial share, reflecting the high prevalence of these conditions requiring immediate ICP management. Hospitals remain the primary end-users, given the intensive nature of neurocritical care. The demand for EVDs made from advanced materials, such as silicone and polyurethane, which offer better biocompatibility and reduced complication rates, also continues to rise, underscoring a market shift towards quality and patient safety.
AI Impact Analysis on External Ventricular Drain Market
Common user questions regarding AI's impact on the External Ventricular Drain Market frequently revolve around the potential for enhanced patient monitoring, predictive analytics for complications, and optimized treatment protocols. Users are keen to understand how AI can improve diagnostic accuracy, reduce human error, and personalize care, while also expressing concerns about data privacy, algorithmic bias, and the validation of AI systems in critical care settings. The overarching expectation is that AI will transform EVD management from a reactive to a proactive approach, leading to better patient outcomes and more efficient resource utilization in neurocritical care units.
- AI-powered algorithms can analyze continuous intracranial pressure (ICP) data, correlating it with other physiological parameters to provide real-time insights into patient status and predict potential complications like shunt malfunction or impending herniation.
- Predictive analytics can forecast the optimal CSF drainage rates and identify patients at higher risk of infection or hemorrhage, enabling proactive interventions and personalized treatment adjustments, thereby enhancing patient safety.
- Automated monitoring systems integrated with EVDs, leveraging AI, can reduce the workload on nursing staff by autonomously flagging critical changes in ICP or CSF flow, allowing clinicians to focus on direct patient care.
- AI can assist in interpreting complex multimodal monitoring data, providing a more comprehensive understanding of brain physiology and guiding clinicians in decisions related to EVD placement, removal, or adjustment.
- Development of AI-driven diagnostic support tools for early identification of hydrocephalus or other conditions requiring EVDs, potentially improving time-to-treatment.
- Optimization of EVD device design and material selection through AI simulations, leading to more biocompatible and effective catheters with reduced infection rates.
- AI can contribute to clinical decision support systems, offering evidence-based recommendations for EVD management protocols, potentially standardizing care and reducing variability in treatment.
DRO & Impact Forces Of External Ventricular Drain Market
The External Ventricular Drain Market is significantly influenced by a confluence of drivers, restraints, and opportunities that collectively shape its trajectory and impact forces. A primary driver is the increasing global prevalence of neurological conditions such as traumatic brain injuries, hydrocephalus, strokes, and brain tumors, all of which often necessitate acute management of intracranial pressure using EVDs. Coupled with an aging global population, which is more susceptible to these conditions, the demand for effective neurocritical care solutions, including EVDs, continues to surge. Technological advancements leading to safer, more precise, and infection-resistant EVD systems also act as powerful market drivers, enhancing clinical utility and patient outcomes.
However, the market faces notable restraints that could impede its growth. A significant concern is the inherent risk of complications associated with EVD placement, particularly infections (ventriculitis) and hemorrhage, which necessitate stringent protocols and can lead to extended hospital stays and increased healthcare costs. The high cost of neurosurgical procedures involving EVDs, coupled with budget constraints in various healthcare systems, can limit adoption, especially in developing regions. Furthermore, the availability of skilled neurosurgeons and specialized neurocritical care units is not uniformly distributed globally, posing a barrier to widespread implementation in underserved areas. These factors combine to create a cautious environment for market expansion.
Despite these challenges, substantial opportunities exist for market players. The development of advanced EVD catheters featuring antimicrobial coatings, designed to significantly reduce infection rates, represents a major growth avenue. Integration of EVD systems with sophisticated smart monitoring technologies that offer continuous, real-time data analysis and remote accessibility could revolutionize patient management. Furthermore, expanding market penetration into emerging economies, where healthcare infrastructure is improving and the burden of neurological diseases is rising, offers untapped potential. Strategic collaborations between device manufacturers, neurosurgical centers, and research institutions to develop innovative solutions and training programs will also be crucial in leveraging these opportunities and driving future market growth, ultimately enhancing patient safety and clinical efficacy.
Segmentation Analysis
The External Ventricular Drain market is meticulously segmented to provide a granular understanding of its various components, enabling stakeholders to identify specific growth areas and tailor their strategies effectively. This segmentation considers different product types, the specific clinical applications for which EVDs are used, the primary end-user settings, and the materials utilized in their construction. Analyzing these segments helps in comprehending market dynamics, competitive landscapes, and evolving customer preferences, leading to more targeted product development and market penetration efforts across diverse healthcare environments.
- By Product Type
- Disposable EVDs
- Reusable EVDs
- By Application
- Hydrocephalus
- Traumatic Brain Injury (TBI)
- Subarachnoid Hemorrhage (SAH)
- Intracerebral Hemorrhage (ICH)
- Other Neurological Conditions
- By End-User
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
- Academic & Research Institutions
- By Material
- Silicone
- Polyurethane
- Other Polymers
- By Age Group
- Pediatric
- Adult
- Geriatric
- By Region
- North America
- Europe
- Asia Pacific (APAC)
- Latin America
- Middle East and Africa (MEA)
Value Chain Analysis For External Ventricular Drain Market
The value chain for the External Ventricular Drain market commences with the upstream activities involving the sourcing and processing of raw materials. This segment primarily consists of suppliers providing high-grade medical plastics such as silicone and polyurethane, metals for connectors, and electronic components for monitoring systems. Manufacturers meticulously select these suppliers based on quality, reliability, and regulatory compliance, as the integrity of raw materials directly impacts the safety and efficacy of the final EVD product. Research and development also play a crucial upstream role, focusing on innovations in material science, design optimization, and advanced manufacturing techniques to enhance device performance and reduce complications.
Midstream activities involve the manufacturing, assembly, and quality control of EVD systems. This stage includes the fabrication of catheters, production of drainage bags, pressure transducers, and the integration of all components into a complete, sterile, and functional system. Strict adherence to medical device regulations (e.g., FDA, CE Mark) is paramount, encompassing rigorous testing, sterilization processes, and packaging to ensure product safety and effectiveness. Companies often invest heavily in advanced manufacturing facilities and automation to achieve economies of scale while maintaining high quality standards, which is critical for medical devices intended for neurocritical applications.
Downstream, the value chain extends to distribution, sales, and post-market support. EVD products are primarily distributed through a network of specialized medical device distributors or direct sales forces to hospitals, neurosurgical units, and intensive care units worldwide. Direct distribution allows for greater control over customer relationships and technical support, while indirect channels leverage the reach and expertise of regional partners. Post-sale services, including training for healthcare professionals on product usage, maintenance, and technical support, are vital for ensuring optimal product performance and patient safety. Effective distribution channels are crucial for timely delivery, especially given the emergency nature of many neurological conditions requiring EVDs.
External Ventricular Drain Market Potential Customers
The primary potential customers and end-users of External Ventricular Drain products are healthcare institutions that specialize in acute neurological care. Hospitals, particularly those with dedicated neurosurgery departments, intensive care units (ICUs), and emergency rooms, represent the largest segment of buyers. These facilities routinely manage patients with severe head injuries, strokes, hydrocephalus, and other critical neurological conditions that necessitate precise intracranial pressure monitoring and cerebrospinal fluid drainage. The demand from hospitals is driven by patient volume, the complexity of cases managed, and the need for advanced medical equipment to provide optimal patient outcomes.
Beyond large hospital systems, specialty neurological clinics and ambulatory surgical centers that perform neurosurgical procedures, albeit often less complex ones, also constitute an important customer base. While these may not require EVDs for acute critical care as frequently as ICUs, they may use them in specific elective or semi-elective procedures where transient CSF drainage or ICP monitoring is required. The increasing focus on specialized care and day-case surgery for certain conditions could gradually expand this customer segment, though EVD usage remains predominantly in high-acuity settings.
Furthermore, academic and research institutions engaged in neurosurgical training, neurological research, and clinical trials also serve as potential customers. These entities acquire EVD systems for educational purposes, simulating clinical scenarios, and for use in research protocols aimed at improving patient care or developing new device technologies. While their procurement volume might be lower than large hospitals, their role in validating new products and shaping future clinical practices makes them strategically important. The long-term relationship with these institutions can influence product adoption and market trends.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 450 million |
| Market Forecast in 2032 | USD 715 million |
| Growth Rate | CAGR 6.8% |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Medtronic plc, B. Braun Melsungen AG, Integra LifeSciences Holdings Corporation, Depuy Synthes (Johnson & Johnson), Raumedic AG, Spiegelberg GmbH & Co. KG, Sophysa SA, Natus Medical Incorporated, Boston Scientific Corporation, Tokibo Co., Ltd., Fuji Systems Corporation, Haiyan Kangyuan Medical Instrument Co., Ltd., Shenyang Headway Medical Equipment Co., Ltd., Vygon S.A., HLL Lifecare Limited, PMT Corporation, Codman Neuro, Mizuho OSI, Deltex Medical Group plc |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
External Ventricular Drain Market Key Technology Landscape
The External Ventricular Drain market is continuously evolving with significant technological advancements aimed at improving patient safety, enhancing device efficacy, and simplifying clinical management. A paramount technological focus is on the development of catheters incorporating antimicrobial coatings. These specialized coatings, often silver-impregnated or antibiotic-eluting, are designed to significantly reduce the risk of ventriculitis and other EVD-related infections, which are among the most serious complications of EVD placement. Research into novel biocompatible materials with inherent antimicrobial properties is also ongoing, promising even safer solutions in the future. These innovations directly address a critical concern in neurocritical care, leading to better patient outcomes and reduced healthcare costs associated with infection management.
Another crucial aspect of the technological landscape involves the integration of advanced monitoring capabilities directly into EVD systems. Modern EVDs often come equipped with highly sensitive pressure transducers that provide continuous, real-time intracranial pressure (ICP) readings, which can be seamlessly integrated with hospital patient monitoring networks. Furthermore, developments include systems that offer multimodal monitoring, combining ICP data with cerebral perfusion pressure (CPP), brain tissue oxygenation, and other vital signs. This holistic data approach provides clinicians with a more comprehensive understanding of the patient's neurological status, enabling more informed and timely interventions for optimal brain protection.
The trend towards "smart" EVD systems is gaining momentum, leveraging digital technologies to enhance functionality and connectivity. This includes the development of EVDs with wireless data transmission capabilities, allowing for remote monitoring and alerts. Such systems can incorporate alarm features for sudden changes in ICP or CSF flow, and potentially integrate with electronic health records (EHR) for streamlined data management and analysis. Future innovations are expected to include AI-driven predictive analytics for identifying at-risk patients and optimizing drainage protocols, further transforming EVD management from a manual, reactive process to a more automated and proactive approach, thereby improving efficiency and patient safety in neurocritical care settings.
Regional Highlights
- North America: The North American market holds a dominant share due to its advanced healthcare infrastructure, high prevalence of neurological disorders, and robust reimbursement policies. The presence of leading market players, high adoption of technologically advanced medical devices, and significant investment in neurocritical care research contribute to its strong position. The United States, in particular, demonstrates high demand for EVDs driven by a large patient pool and sophisticated diagnostic capabilities.
- Europe: Europe represents a mature market with steady growth, characterized by strong public healthcare systems and a focus on research and development in medical technology. Countries like Germany, the UK, and France are significant contributors, propelled by an aging population and increasing incidence of neurodegenerative diseases and trauma. Stringent regulatory frameworks ensure high-quality medical device standards, fostering trust and adoption.
- Asia Pacific (APAC): The Asia Pacific region is projected to exhibit the fastest growth rate during the forecast period. This growth is attributed to improving healthcare infrastructure, rising healthcare expenditure, increasing medical tourism, and a large patient population susceptible to neurological conditions. Emerging economies such as China and India are investing heavily in upgrading their critical care facilities and increasing access to advanced medical treatments, presenting substantial opportunities for market expansion.
- Latin America: The Latin American market is an emerging region for EVDs, showing promising growth driven by increasing awareness of neurological conditions and gradual improvements in healthcare access and infrastructure. Countries like Brazil and Mexico are leading the adoption, albeit with challenges related to healthcare spending and availability of specialized neurosurgical expertise. Investment in public health initiatives and medical education is crucial for sustained market development.
- Middle East and Africa (MEA): The MEA market is still nascent but poised for growth, largely due to ongoing healthcare infrastructure development projects, increasing government spending on healthcare, and a rising prevalence of non-communicable diseases, including neurological disorders. Economic diversification efforts and medical tourism initiatives in countries like UAE and Saudi Arabia are creating demand for advanced medical devices, though access and affordability remain key considerations across the broader region.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the External Ventricular Drain Market.- Medtronic plc
- B. Braun Melsungen AG
- Integra LifeSciences Holdings Corporation
- Depuy Synthes (Johnson & Johnson)
- Raumedic AG
- Spiegelberg GmbH & Co. KG
- Sophysa SA
- Natus Medical Incorporated
- Boston Scientific Corporation
- Tokibo Co., Ltd.
- Fuji Systems Corporation
- Haiyan Kangyuan Medical Instrument Co., Ltd.
- Shenyang Headway Medical Equipment Co., Ltd.
- Vygon S.A.
- HLL Lifecare Limited
- PMT Corporation
- Codman Neuro
- Mizuho OSI
- Deltex Medical Group plc
- Dispomedica GmbH
Frequently Asked Questions
What is an External Ventricular Drain (EVD) and why is it used?
An External Ventricular Drain (EVD) is a neurosurgical device used to divert cerebrospinal fluid (CSF) from the brain's ventricles to an external collection system. It is primarily used to manage elevated intracranial pressure (ICP) in conditions such as hydrocephalus, traumatic brain injury, or intracranial hemorrhage, and for diagnostic CSF sampling.
What are the main risks associated with EVD placement?
The primary risks associated with EVD placement include infection, particularly ventriculitis, and hemorrhage. Other potential complications involve catheter malfunction, obstruction, or displacement, which can lead to inadequate CSF drainage or inaccurate ICP monitoring. Strict aseptic techniques and careful patient management are crucial to mitigate these risks.
How is the External Ventricular Drain market segmented by application?
The External Ventricular Drain market is segmented by application into key areas such as Hydrocephalus, Traumatic Brain Injury (TBI), Subarachnoid Hemorrhage (SAH), Intracerebral Hemorrhage (ICH), and Other Neurological Conditions. Each segment represents distinct patient populations and clinical needs driving demand for EVD systems.
What technological advancements are impacting the EVD market?
Key technological advancements impacting the EVD market include the development of antimicrobial-coated catheters to reduce infection rates, integration of advanced pressure transducers for real-time ICP monitoring, and the emergence of "smart" EVD systems with wireless connectivity and predictive analytics capabilities for enhanced patient management and safety.
Which region is expected to show the highest growth in the EVD market?
The Asia Pacific (APAC) region is projected to exhibit the highest growth rate in the External Ventricular Drain market during the forecast period. This growth is driven by improving healthcare infrastructure, increasing healthcare expenditure, a large and aging patient population, and rising awareness regarding neurological conditions across developing economies in the region.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
