Fetal Monitoring Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427867 | Date : Oct, 2025 | Pages : 248 | Region : Global | Publisher : MRU
Fetal Monitoring Market Size
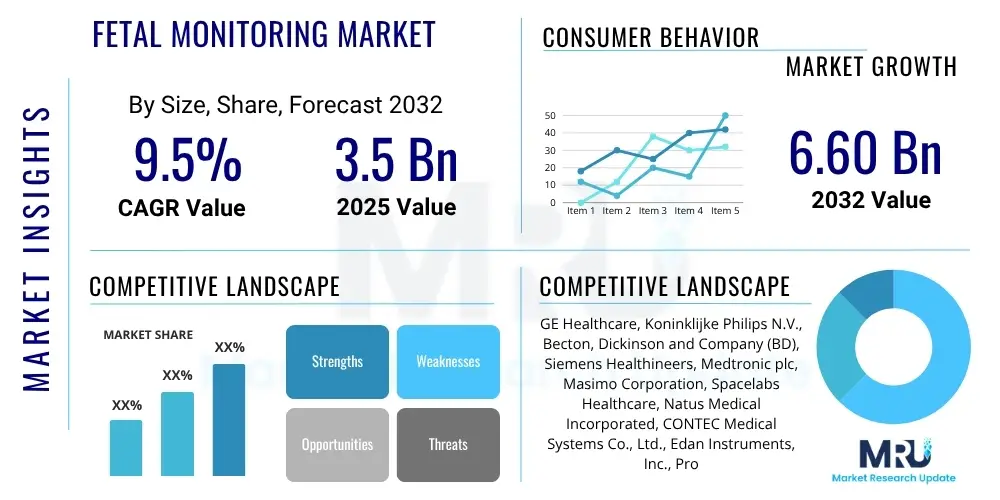
The Fetal Monitoring Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 9.5% between 2025 and 2032. The market is estimated at 3.5 Billion USD in 2025 and is projected to reach 6.60 Billion USD by the end of the forecast period in 2032.
Fetal Monitoring Market introduction
The Fetal Monitoring Market represents a cornerstone of modern obstetric care, encompassing a comprehensive array of technologies and services designed to assess the health and well-being of the fetus throughout pregnancy and during labor. These sophisticated systems play a vital role in identifying potential complications such as fetal distress, hypoxia, and congenital anomalies, thereby enabling timely medical interventions that significantly improve both maternal and neonatal outcomes. The scope of fetal monitoring extends from routine antenatal check-ups for low-risk pregnancies to intensive surveillance for high-risk cases, reflecting its indispensable nature in ensuring safe deliveries and healthy beginnings. Continuous innovation in this sector is driven by the imperative to enhance diagnostic accuracy, reduce invasiveness, and improve patient comfort, contributing to a dynamic and evolving market landscape.
Fetal monitoring products can be broadly categorized into external and internal methods, each serving distinct clinical needs. External monitoring, the most common approach, typically involves non-invasive devices such as Doppler ultrasound systems and cardiotocographs (CTGs) that measure fetal heart rate (FHR) and uterine contractions (UC) via transducers placed on the mother's abdomen. These devices are widely utilized during antenatal visits and for intrapartum surveillance in low-risk scenarios. Conversely, internal fetal monitoring, which includes direct fetal electrocardiogram (ECG) electrodes placed on the fetal scalp or intrauterine pressure catheters (IUPCs) to measure contraction strength, offers highly precise data and is generally reserved for high-risk pregnancies or when external monitoring provides insufficient information during labor. The continuous development of wireless and portable devices further expands the utility and accessibility of these monitoring solutions, promoting greater maternal mobility and comfort.
The applications of fetal monitoring are extensive, ranging from routine prenatal assessments, where intermittent monitoring helps establish baseline fetal health, to critical intrapartum surveillance, where continuous monitoring guides decisions regarding labor progression and potential delivery interventions. Benefits derived from effective fetal monitoring are profound, including the early detection of fetal hypoxia, the ability to prevent adverse birth outcomes such as cerebral palsy and stillbirths, and overall improvements in perinatal mortality and morbidity rates. Key driving factors propelling market growth include a global rise in birth rates, particularly in developing economies, and an increasing prevalence of high-risk pregnancies attributable to factors such as advancing maternal age, the growing incidence of chronic maternal health conditions like gestational diabetes and hypertension, and the widespread use of assisted reproductive technologies. Furthermore, technological advancements leading to more accurate, user-friendly, and integrated monitoring platforms, coupled with increasing awareness among healthcare providers and expectant parents regarding the importance of comprehensive fetal surveillance, continue to fuel market expansion.
Fetal Monitoring Market Executive Summary
The Fetal Monitoring Market is experiencing robust growth, propelled by a confluence of demographic shifts, technological advancements, and evolving healthcare paradigms. Key business trends indicate a strong move towards non-invasive, portable, and user-friendly monitoring solutions, catering to the rising demand for home-based care and remote monitoring, particularly accelerated by global health challenges that emphasized the need for telehealth services. Companies are heavily investing in research and development to integrate artificial intelligence (AI) and machine learning (ML) algorithms into their devices, enhancing data interpretation capabilities, improving diagnostic accuracy, and providing predictive analytics for early detection of potential complications. The competitive landscape is characterized by strategic collaborations, mergers, and acquisitions aimed at expanding product portfolios, market reach, and technological expertise, ensuring a dynamic environment of continuous innovation and market penetration.
Regional trends reveal significant disparities and growth opportunities across various geographies. North America and Europe continue to be dominant markets, driven by well-established healthcare infrastructures, high healthcare expenditure, and rapid adoption of advanced monitoring technologies. These regions are characterized by a strong emphasis on clinical guidelines and a demand for sophisticated, high-precision devices. Conversely, the Asia Pacific (APAC) region is emerging as the fastest-growing market, primarily due to its large population base, increasing birth rates, improving healthcare access and infrastructure, and rising disposable incomes that enable greater investment in maternal and child health. Latin America, the Middle East, and Africa are also witnessing substantial growth, albeit from a smaller base, fueled by increasing awareness, governmental initiatives to improve maternal healthcare, and the expansion of healthcare facilities, presenting lucrative opportunities for market players to introduce cost-effective and adaptable solutions tailored to regional needs.
Segmentation trends within the fetal monitoring market underscore a clear shift towards advanced, integrated solutions. The portable and wireless device segments are experiencing accelerated growth, driven by their convenience, ability to facilitate maternal mobility, and suitability for remote monitoring applications. Non-invasive methods continue to dominate, reflecting a preference for patient comfort and reduced procedural risks, though invasive techniques remain critical for high-risk scenarios requiring precise data. In terms of end-users, hospitals remain the largest segment, but clinics, birthing centers, and home care settings are rapidly expanding their adoption of fetal monitoring devices, especially portable and telehealth-integrated systems. This diversification across end-user settings is broadening market accessibility and allowing for continuous, personalized care. The integration of software and services, beyond just hardware, is also a significant trend, offering comprehensive solutions for data management, analysis, and clinical decision support, transforming the market from purely device-centric to solution-oriented.
AI Impact Analysis on Fetal Monitoring Market
The integration of Artificial Intelligence (AI) into the Fetal Monitoring Market is a transformative development, addressing common user concerns regarding accuracy, efficiency, and the interpretive challenges associated with traditional fetal monitoring data. Users frequently inquire about AI's potential to reduce false positive rates in cardiotocography (CTG) interpretations, enhance the early detection of fetal distress, and streamline clinical workflows. They also express interest in how AI can support remote monitoring by autonomously analyzing complex data patterns and flagging anomalies for healthcare providers, thereby extending continuous surveillance beyond traditional hospital settings. There are also queries about the safety and reliability of AI algorithms, their validation processes, and the ethical implications of relying on automated systems for critical diagnostic decisions. Users generally anticipate that AI will provide more objective, consistent, and actionable insights, augmenting clinician capabilities rather than replacing them, ultimately leading to improved patient outcomes and a reduction in perinatal morbidity.
AI's influence is multifaceted, impacting various stages of fetal monitoring from data acquisition to clinical decision support. The primary expectation is that AI algorithms can sift through vast amounts of physiological data—including fetal heart rate, uterine contractions, and maternal vital signs—to identify subtle patterns and trends that might be imperceptible to the human eye. This capability is poised to significantly enhance the accuracy of diagnosing conditions like hypoxia or placental insufficiency much earlier, allowing for preemptive interventions. Furthermore, AI can personalize monitoring protocols by adapting to individual maternal and fetal profiles, reducing unnecessary interventions for low-risk cases while intensifying surveillance for high-risk pregnancies. The ability of AI to learn from large datasets of both normal and abnormal outcomes promises to create more robust and predictive diagnostic tools, ultimately fostering a proactive approach to obstetric care and empowering clinicians with enhanced analytical capabilities.
The long-term vision for AI in fetal monitoring extends to creating fully integrated, smart monitoring systems that not only collect and analyze data but also provide real-time, evidence-based recommendations for clinical action. This includes developing predictive models for labor progression, identifying optimal timing for interventions, and even assisting in postpartum follow-up by tracking recovery patterns. While the potential benefits are immense, the market is also grappling with the need for rigorous validation of AI algorithms, ensuring their reliability across diverse patient populations, and establishing clear regulatory frameworks to govern their deployment. Addressing concerns about data privacy, algorithmic bias, and the necessity for human oversight remains paramount. As AI technologies mature and become more seamlessly integrated into existing healthcare IT infrastructures, they are set to revolutionize fetal monitoring by making it more precise, predictive, accessible, and ultimately, safer for mothers and infants globally.
- Enhanced Fetal Heart Rate (FHR) Pattern Recognition: AI algorithms can detect subtle FHR decelerations, accelerations, and variability changes with higher precision than manual interpretation, improving early detection of fetal distress.
- Reduced False Positives/Negatives: By analyzing complex datasets, AI can help clinicians differentiate between benign and pathological FHR patterns, potentially reducing unnecessary interventions (e.g., C-sections) caused by false alarms.
- Predictive Analytics: AI-powered systems can forecast potential adverse events by identifying high-risk trends in real-time, enabling proactive clinical management and personalized care plans.
- Remote Monitoring and Telehealth Integration: AI facilitates the analysis of data from home-based or wearable fetal monitors, enabling continuous surveillance and timely alerts for clinicians without requiring constant physical presence.
- Workflow Optimization and Decision Support: AI assists healthcare professionals by automating data interpretation, generating concise summaries, and providing evidence-based recommendations, freeing up clinicians for direct patient care.
- Data Integration and Interpretation: AI can synthesize data from multiple sources (e.g., CTG, ultrasound, maternal vitals) to provide a holistic view of fetal well-being, enhancing comprehensive diagnostic capabilities.
- Personalized Risk Assessment: Leveraging patient-specific data, AI can develop individualized risk profiles, tailoring monitoring strategies and intervention thresholds to optimize outcomes for each mother and fetus.
DRO & Impact Forces Of Fetal Monitoring Market
The Fetal Monitoring Market is profoundly shaped by a dynamic interplay of driving forces, inherent restraints, emergent opportunities, and influential external forces. A primary driver is the global increase in birth rates, particularly in populous developing countries, coupled with a rising prevalence of high-risk pregnancies stemming from factors like delayed childbearing, increasing rates of maternal chronic conditions suchos as diabetes and hypertension, and complications arising from assisted reproductive technologies. These demographic and epidemiological shifts necessitate more vigilant and sophisticated fetal surveillance. Furthermore, continuous technological advancements, including the development of wireless, portable, and AI-integrated monitoring devices, significantly enhance diagnostic accuracy, user convenience, and remote care capabilities, thereby stimulating market growth. Growing awareness among healthcare providers and expectant parents about the critical importance of early detection of fetal complications also plays a crucial role in driving the adoption of advanced monitoring solutions.
Despite these significant drivers, the market faces several notable restraints. The high cost associated with advanced fetal monitoring equipment, particularly for continuous and sophisticated systems, poses a significant barrier to adoption, especially in resource-limited settings. This cost extends beyond initial purchase to maintenance, consumables, and the training of skilled personnel. A shortage of adequately trained healthcare professionals capable of operating and accurately interpreting complex fetal monitoring data presents another challenge, leading to potential misdiagnoses or underutilization of advanced features. Moreover, regulatory complexities and stringent approval processes for novel medical devices can delay market entry and increase development costs, while concerns regarding the accuracy of some monitoring techniques, including issues like false positives or negatives, can lead to patient anxiety or unnecessary interventions. These factors collectively temper the market's growth potential and necessitate strategic approaches to overcome them.
Amidst these challenges, significant opportunities are emerging that are poised to reshape the market. The expansion of telehealth and remote monitoring services, accelerated by recent global health events, offers a substantial avenue for growth, enabling continuous fetal surveillance outside traditional clinical environments and improving accessibility, especially in rural or underserved areas. The development of more cost-effective, portable, and user-friendly devices specifically tailored for home use represents a lucrative opportunity to cater to a broader consumer base. Furthermore, advancements in data analytics and artificial intelligence present opportunities for developing predictive algorithms that can offer more precise and personalized risk assessments, leading to more targeted interventions. The vast untapped markets in emerging economies, coupled with increasing healthcare expenditure and improving infrastructure, provide fertile ground for market expansion, particularly for innovative solutions that balance advanced functionality with affordability and ease of use, positioning the market for sustained innovation and global reach.
Segmentation Analysis
The Fetal Monitoring Market is intricately segmented across various dimensions, providing a comprehensive view of its diverse product offerings, applications, and end-user base. This segmentation analysis is crucial for understanding market dynamics, identifying specific growth opportunities, and tailoring strategies to cater to distinct market needs. The market is primarily segmented by product type (hardware, software, services), portability (portable, non-portable), method (invasive, non-invasive), application (ante-partum, intra-partum), and end-user (hospitals, clinics, home care settings). Each segment exhibits unique growth patterns and demand drivers, reflecting the evolving landscape of maternal and fetal healthcare. Understanding these granular segments allows stakeholders to pinpoint areas of high potential and develop targeted solutions that address specific clinical requirements and patient preferences across the globe.
- By Product Type:
- Hardware: This segment includes the physical devices used for fetal monitoring, such as Cardiotocographs (CTGs), Doppler ultrasound devices, fetal ECG monitors, and intrauterine pressure catheters. It accounts for the largest share due to initial investment.
- Software: Comprises applications for data analysis, interpretation, storage, and integration with hospital information systems (HIS) or electronic health records (EHR). Growth is driven by the need for advanced analytics and remote data management.
- Services: Encompasses maintenance, installation, training, and technical support services for fetal monitoring equipment, crucial for optimal device performance and longevity.
- By Portability:
- Portable Devices: Includes handheld Doppler devices, wireless CTGs, and wearable monitors designed for mobility and use outside traditional clinical settings, favored for home care and telehealth.
- Non-Portable Devices: Primarily traditional, cart-based CTGs and large ultrasound machines used in hospitals and specialized clinics, offering comprehensive monitoring capabilities.
- By Method:
- Non-Invasive Monitoring: Dominant segment, utilizing external transducers for FHR and UC monitoring (e.g., external CTG, Doppler ultrasound), preferred for patient comfort and reduced risk.
- Invasive Monitoring: Involves internal electrodes for FHR (e.g., fetal scalp electrode) and intrauterine pressure catheters for UC, used in high-risk pregnancies during labor for higher accuracy.
- By Application:
- Antepartum Monitoring: Focuses on fetal health assessment before labor, including routine check-ups, non-stress tests (NSTs), and biophysical profiles, particularly for high-risk pregnancies.
- Intrapartum Monitoring: Involves continuous surveillance during labor to detect fetal distress, guide delivery decisions, and ensure maternal and fetal safety.
- By End-User:
- Hospitals: The largest end-user segment, comprising maternity wards, labor & delivery units, and neonatal intensive care units, due to the volume of births and complexity of cases.
- Clinics and Birthing Centers: Growing segment, adopting portable and less invasive devices for routine care and low-risk deliveries.
- Home Care Settings: Rapidly expanding segment driven by telehealth, wearable technology, and the demand for convenient, continuous monitoring for expecting mothers at home.
Value Chain Analysis For Fetal Monitoring Market
The value chain for the Fetal Monitoring Market is a complex and interconnected network, beginning with extensive research and development and extending through to the end-users. At the upstream end, the chain is dominated by raw material suppliers providing specialized components such as ultrasonic transducers, sensors, microprocessors, and advanced display technologies. These suppliers are critical for ensuring the quality and functionality of the final medical devices. Research and development activities, encompassing clinical trials, software development for algorithms, and engineering design, represent a significant value-adding stage, where innovations in non-invasive techniques, wireless capabilities, and AI integration are conceived. Collaborations between academic institutions, medical device companies, and technology firms are commonplace in this phase, aiming to enhance product efficacy, safety, and user experience. This foundational stage dictates the technological sophistication and competitive edge of market offerings, emphasizing continuous investment in innovation to meet evolving clinical demands.
Midstream activities involve the manufacturing and assembly of fetal monitoring devices. This stage is characterized by stringent quality control processes and compliance with international medical device regulations, such as FDA approvals and CE markings. Companies often operate highly specialized production facilities, utilizing advanced manufacturing techniques to ensure precision, reliability, and scalability. Following manufacturing, the distribution channel plays a crucial role in delivering these sophisticated devices to healthcare providers globally. Distribution can be bifurcated into direct and indirect channels. Direct distribution involves manufacturers selling directly to large hospital networks, government health programs, or key opinion leaders, allowing for greater control over sales, pricing, and customer relationships. This channel is often utilized for high-value, complex systems requiring specialized installation and training. Manufacturers may also employ direct sales teams to engage with major clients, offering customized solutions and comprehensive support packages, strengthening client loyalty and brand presence.
Indirect distribution, conversely, leverages a network of third-party distributors, wholesalers, and medical equipment resellers. This approach is particularly effective for reaching smaller clinics, independent practitioners, and expanding into geographically diverse or emerging markets where direct presence may not be feasible or cost-effective. These distributors often have established logistics networks, local market expertise, and existing relationships with healthcare providers, enabling broader market penetration and efficient supply chain management. Post-sales services, including installation, maintenance, repair, and ongoing technical support, form a crucial part of the downstream value chain, ensuring device longevity and optimal performance. Furthermore, training and education for healthcare professionals on the effective use and interpretation of fetal monitoring data add significant value, ensuring that the advanced capabilities of these devices are fully realized. Ultimately, the efficiency and effectiveness of this entire value chain are pivotal in ensuring that innovative fetal monitoring solutions reach those who need them most, thereby improving maternal and fetal health outcomes worldwide.
Fetal Monitoring Market Potential Customers
The Fetal Monitoring Market serves a diverse range of potential customers, all unified by the common objective of ensuring maternal and fetal well-being during pregnancy and childbirth. Hospitals represent the largest and most critical segment of end-users. Within hospitals, the primary departments utilizing fetal monitoring equipment include labor and delivery units, maternity wards, prenatal care clinics, and neonatal intensive care units (NICUs). These institutions require a comprehensive suite of monitoring devices, from advanced non-portable CTGs for continuous intrapartum surveillance to portable ultrasound systems for antenatal assessments, catering to a high volume of diverse cases, including high-risk pregnancies requiring intensive monitoring. Hospitals prioritize accuracy, reliability, integration with existing electronic health records (EHR) systems, and robust after-sales support to manage their complex patient populations and ensure seamless clinical workflows, making them central to the market's demand landscape.
Beyond large hospital systems, a significant and growing customer base includes smaller specialized healthcare facilities such as private clinics, obstetrician-gynecologist (OB/GYN) offices, and independent birthing centers. These establishments primarily seek portable, user-friendly, and cost-effective fetal monitoring solutions for routine antenatal check-ups and low-risk deliveries. The demand in these settings is often for devices that offer a balance between clinical efficacy and convenience, with an increasing preference for wireless and telehealth-compatible systems that enhance patient experience and operational efficiency. The ability of these smaller clinics to provide continuous, yet less restrictive, monitoring options helps to attract patients seeking personalized care in a more intimate setting, thereby driving the adoption of flexible monitoring technologies that support ambulatory care models.
Emerging as a rapidly expanding customer segment are individual expecting parents and home care providers, driven by the accelerating trend towards remote monitoring and home-based care. With technological advancements making fetal monitoring devices more compact, affordable, and easier to operate, there is a burgeoning market for consumer-grade or prescription-based home fetal monitors. These devices enable expecting mothers, especially those with high-risk pregnancies or geographical limitations, to conduct regular fetal health checks from the comfort of their homes, transmitting data to healthcare providers for remote assessment. This segment values ease of use, connectivity, data security, and the psychological comfort of continuous monitoring without frequent hospital visits. The market is also increasingly targeting telehealth platforms and remote diagnostic service providers who integrate fetal monitoring data into their comprehensive virtual care offerings, further broadening the customer base beyond traditional clinical settings and democratizing access to essential maternal and fetal healthcare.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | 3.5 Billion USD |
| Market Forecast in 2032 | 6.60 Billion USD |
| Growth Rate | 9.5% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | GE Healthcare, Koninklijke Philips N.V., Becton, Dickinson and Company (BD), Siemens Healthineers, Medtronic plc, Masimo Corporation, Spacelabs Healthcare, Natus Medical Incorporated, CONTEC Medical Systems Co., Ltd., Edan Instruments, Inc., Promed Group, CooperSurgical, Inc., Mindray Medical International Limited, Fujifilm Holdings Corporation, Vyaire Medical, Inc. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Fetal Monitoring Market Key Technology Landscape
The Fetal Monitoring Market is characterized by a rapidly evolving technological landscape, driven by the continuous quest for enhanced accuracy, reduced invasiveness, and improved patient convenience. At the core of existing technologies are Doppler ultrasound and Cardiotocography (CTG) systems. Doppler ultrasound utilizes sound waves to detect and measure fetal heart rate (FHR), providing non-invasive, real-time information. CTG, on the other hand, simultaneously monitors FHR and uterine contractions (UC) to assess fetal well-being, traditionally through external transducers placed on the maternal abdomen. Recent advancements have focused on refining these core technologies, including the development of advanced signal processing algorithms that enhance data clarity and reduce artifacts, leading to more reliable diagnostic outputs and minimizing the incidence of false positives or negatives, which were common limitations in earlier generations of devices. This continuous refinement forms the bedrock upon which newer innovations are built, ensuring a solid foundation for future technological leaps.
A significant trend in the technological landscape is the shift towards wireless and portable monitoring solutions. Traditional wired systems often restricted maternal mobility, which can be a comfort issue during labor. Wireless CTG systems, incorporating Bluetooth or Wi-Fi connectivity, allow mothers greater freedom of movement while ensuring continuous data transmission to healthcare providers. Similarly, advancements in miniaturization have led to the development of highly portable and even wearable fetal monitors, often integrated with smartphone applications for easy data display and sharing. These innovations are critical for expanding access to fetal monitoring beyond hospital settings, facilitating remote monitoring, and enabling telehealth services. The move towards non-invasive and discreet devices further addresses patient preferences, making monitoring less intrusive and more integrated into daily life, which is particularly relevant for routine antenatal care and home-based surveillance, thereby enhancing patient compliance and comfort significantly.
The most transformative technological advancements currently reshaping the market involve the integration of artificial intelligence (AI) and machine learning (ML) algorithms. These advanced analytical tools are being employed to automate the interpretation of complex CTG traces, detect subtle patterns indicative of fetal distress, and provide predictive insights into potential complications. AI-powered software solutions can process vast amounts of physiological data, learning from clinical outcomes to improve diagnostic accuracy and offer decision support to clinicians, thereby augmenting human expertise. Furthermore, the development of multi-parameter monitoring systems that integrate data from FHR, UC, maternal vital signs (e.g., blood pressure, oxygen saturation), and even fetal oxygen saturation (SfO2) sensors is providing a more holistic view of fetal health. Telemonitoring capabilities, secure cloud storage for data, and interoperability with electronic health record (EHR) systems are also key technological enablers, ensuring seamless data flow, efficient clinical workflows, and collaborative care models. These sophisticated technologies collectively contribute to more precise, proactive, and patient-centric fetal monitoring, revolutionizing obstetric care.
Regional Highlights
The Fetal Monitoring Market exhibits distinct regional dynamics, influenced by varying healthcare infrastructures, economic conditions, birth rates, and adoption rates of advanced medical technologies. North America, particularly the United States and Canada, stands as a leading region due to high healthcare expenditure, sophisticated medical facilities, and widespread awareness regarding maternal and fetal health. The rapid adoption of advanced monitoring technologies, coupled with a strong emphasis on continuous innovation and a favorable regulatory environment, drives market growth. Europe also holds a significant market share, propelled by an aging maternal population leading to more high-risk pregnancies, robust healthcare systems, and increasing government investments in improving perinatal care. Countries like Germany, the UK, and France are at the forefront of adopting innovative fetal monitoring solutions and implementing advanced clinical guidelines, ensuring comprehensive care for expectant mothers.
- North America: Dominant market share attributed to advanced healthcare infrastructure, high adoption of modern medical technologies, increasing prevalence of high-risk pregnancies, and substantial R&D investments. Key countries include the United States and Canada, which have established regulatory frameworks and strong market players.
- Europe: Significant market presence driven by an increasing geriatric maternal population, robust healthcare spending, high awareness of fetal well-being, and government initiatives promoting advanced obstetric care. Germany, the UK, and France are major contributors, characterized by technological early adoption and stringent quality standards.
- Asia Pacific (APAC): Fastest-growing region, fueled by its vast population base, rising birth rates, improving healthcare access and infrastructure, and increasing disposable incomes. Countries like China, India, and Japan are witnessing substantial investments in maternal healthcare, coupled with a growing demand for portable and affordable monitoring solutions.
- Latin America: Emerging market with steady growth, primarily due to increasing awareness regarding prenatal care, improving healthcare infrastructure, and government efforts to reduce maternal and infant mortality rates. Brazil and Mexico are key markets, characterized by a growing middle class and expanding access to modern medical facilities.
- Middle East and Africa (MEA): Witnessing gradual growth, driven by increasing healthcare expenditure, rising birth rates, improving access to medical technologies in urban centers, and governmental focus on enhancing maternal and child health outcomes. Countries like Saudi Arabia, UAE, and South Africa are leading the adoption of advanced fetal monitoring systems in the region.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Fetal Monitoring Market.- GE Healthcare
- Koninklijke Philips N.V.
- Becton, Dickinson and Company (BD)
- Siemens Healthineers
- Medtronic plc
- Masimo Corporation
- Spacelabs Healthcare
- Natus Medical Incorporated
- CONTEC Medical Systems Co., Ltd.
- Edan Instruments, Inc.
- Promed Group
- CooperSurgical, Inc.
- Mindray Medical International Limited
- Fujifilm Holdings Corporation
- Vyaire Medical, Inc.
Frequently Asked Questions
What is fetal monitoring used for?
Fetal monitoring is primarily used to assess the health and well-being of a fetus during pregnancy and labor. It helps detect potential complications such as fetal distress, oxygen deprivation (hypoxia), irregular heartbeats, and other anomalies early, enabling healthcare providers to intervene promptly to ensure safer deliveries and improved maternal and neonatal outcomes. It is crucial for both routine checks and high-risk pregnancies.
What are the different types of fetal monitoring?
Fetal monitoring can be broadly categorized into external and internal methods. External monitoring, which is non-invasive, uses devices like Doppler ultrasound and Cardiotocographs (CTGs) placed on the mother's abdomen to measure fetal heart rate and uterine contractions. Internal monitoring, more invasive, involves placing electrodes directly on the fetal scalp or an intrauterine pressure catheter inside the uterus, typically used during labor for high-risk cases requiring precise data.
Is fetal monitoring safe?
Yes, fetal monitoring is generally considered safe. External monitoring methods are non-invasive and pose no known risks to the mother or fetus. Internal monitoring, while invasive, is performed by trained medical professionals under sterile conditions, and the risks are minimal compared to the benefits of obtaining critical fetal health information, especially in high-risk scenarios. Healthcare providers always weigh the benefits against any potential, albeit rare, risks.
How has technology changed fetal monitoring?
Technology has revolutionized fetal monitoring by introducing wireless, portable, and wearable devices that enhance maternal mobility and enable remote monitoring via telehealth. Advanced software and artificial intelligence (AI) now assist in interpreting complex data patterns, improving diagnostic accuracy, reducing false alarms, and providing predictive insights. These advancements have made monitoring more precise, accessible, user-friendly, and integrated into modern healthcare systems.
Who needs fetal monitoring?
Fetal monitoring is recommended for all pregnant individuals to varying degrees, from routine intermittent checks for low-risk pregnancies to continuous surveillance for high-risk pregnancies. High-risk factors include advanced maternal age, chronic conditions like diabetes or hypertension, previous pregnancy complications, multiple gestations, or suspected fetal growth restriction. It is a standard procedure during labor for most deliveries to ensure fetal well-being.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
