Gastrointestinal Stents Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 430560 | Date : Nov, 2025 | Pages : 241 | Region : Global | Publisher : MRU
Gastrointestinal Stents Market Size
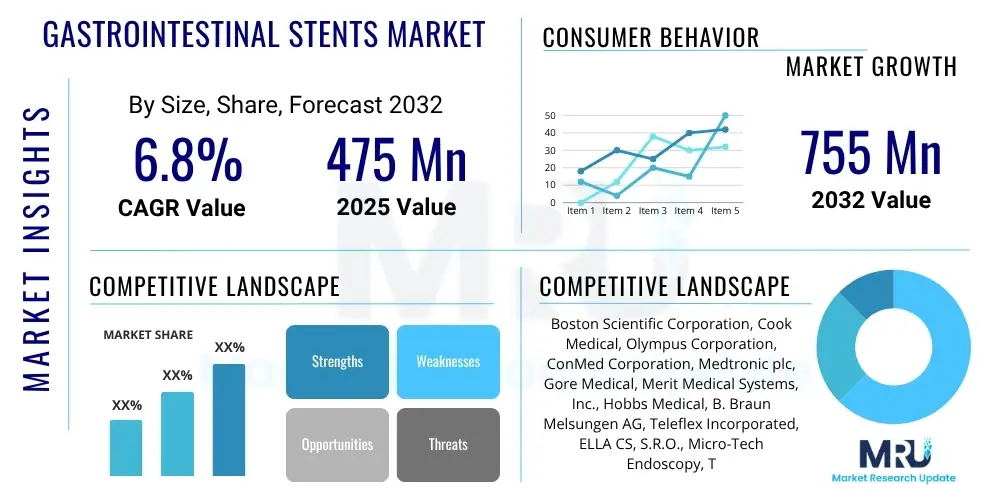
The Gastrointestinal Stents Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2025 and 2032. The market is estimated at $475 Million in 2025 and is projected to reach $755 Million by the end of the forecast period in 2032.
Gastrointestinal Stents Market introduction
The Gastrointestinal Stents Market encompasses a range of medical devices designed to restore patency in obstructed portions of the gastrointestinal tract, including the esophagus, duodenum, colon, and biliary and pancreatic ducts. These stents are critical for alleviating symptoms such as dysphagia, nausea, vomiting, and jaundice caused by malignant tumors, benign strictures, or other pathological conditions. The primary benefit of using GI stents lies in their ability to provide immediate symptomatic relief, improve patient quality of life, and often serve as a less invasive alternative to surgical interventions, particularly in palliative care settings or for patients who are not surgical candidates.
The product portfolio within this market includes various types of stents classified by their location of deployment, material composition, and design. Self-expandable metallic stents (SEMS) are widely adopted due to their radial force, flexibility, and durability, offering sustained patency. Plastic stents, while less durable, are often used for temporary applications or in less severe obstructions. Emerging innovations focus on biodegradable stents, drug-eluting stents to prevent restenosis, and stents with anti-reflux mechanisms, addressing critical clinical needs and expanding their therapeutic utility. These devices play a pivotal role in managing a growing burden of gastrointestinal diseases.
Major applications for gastrointestinal stents include the management of esophageal cancer, pancreatic cancer, colorectal cancer, benign esophageal strictures, common bile duct obstructions, and gastroduodenal obstructions. Their utility extends across both palliative and curative care pathways, often serving as a bridge to surgery or a definitive treatment for symptom management. The market is significantly driven by factors such as the increasing global prevalence of gastrointestinal cancers and benign strictures, the rising geriatric population more susceptible to these conditions, and a strong preference for minimally invasive endoscopic procedures due to their reduced recovery times and lower complication rates compared to open surgery. Continuous advancements in material science and stent design further propel market expansion by enhancing device performance and broadening indications.
Gastrointestinal Stents Market Executive Summary
The Gastrointestinal Stents Market is poised for substantial growth, driven by a confluence of evolving business trends, regional dynamics, and segment-specific innovations. A prominent business trend includes strategic collaborations and acquisitions among key players, aiming to consolidate market share, diversify product portfolios, and enhance research and development capabilities, particularly in advanced materials and smart stent technologies. There is a discernible shift towards value-based healthcare, prompting manufacturers to focus on cost-effective solutions while maintaining high clinical efficacy. Furthermore, increasing investment in clinical trials to expand indications and gather robust evidence for stent performance is shaping market competition. The global market is also witnessing a trend towards personalized medicine, where stent selection and deployment are increasingly tailored to individual patient anatomy and disease pathology, facilitated by advanced imaging and diagnostic tools.
Regionally, North America and Europe currently dominate the market, attributed to their well-established healthcare infrastructure, high awareness of advanced endoscopic procedures, and significant healthcare expenditure. However, the Asia Pacific region is emerging as the fastest-growing market, propelled by rapidly improving healthcare access, increasing prevalence of lifestyle-related gastrointestinal disorders, and a burgeoning elderly population. Latin America, the Middle East, and Africa are also showing promising growth, albeit from a smaller base, as these regions experience enhancements in medical facilities and a greater adoption of advanced medical technologies. Local manufacturing and distribution partnerships are becoming crucial for market penetration in these developing regions, adapting products to local healthcare needs and economic conditions.
Segmentation trends highlight the continued dominance of self-expandable metallic stents (SEMS) across various applications due to their superior radial force and long-term patency. However, the market is experiencing a significant surge in demand for biodegradable stents, which offer the advantage of gradual degradation, eliminating the need for a second endoscopic procedure for removal and reducing long-term complication risks. Drug-eluting stents are also gaining traction, particularly for preventing restenosis in benign strictures. Application-wise, malignant obstructions, especially those caused by pancreaticobiliary and esophageal cancers, constitute the largest segment. The end-user landscape sees hospitals as the primary consumers, although ambulatory surgical centers are increasingly adopting GI stent procedures for their cost-effectiveness and efficiency, particularly for elective cases. These segment trends underscore a market driven by innovation, patient comfort, and clinical outcomes.
AI Impact Analysis on Gastrointestinal Stents Market
Users frequently inquire about the transformative potential of Artificial intelligence (AI) in revolutionizing the Gastrointestinal Stents Market. Common questions revolve around how AI can enhance diagnostic accuracy for identifying obstructions, improve the precision of stent placement procedures, optimize stent design for better patient outcomes, and contribute to personalized treatment strategies. There is also significant interest in AI's role in predicting complications post-stent insertion, facilitating early intervention, and streamlining the research and development process for new stent technologies. Concerns often include data privacy, the interoperability of AI systems with existing medical infrastructure, the need for robust validation, and the regulatory challenges associated with integrating AI-powered solutions into medical devices and clinical workflows. Users seek clarity on how AI can move beyond theoretical applications to deliver tangible clinical and operational benefits within the GI stent landscape, particularly in reducing procedural risks and improving long-term patency.
- Enhanced diagnostic accuracy through AI-powered image analysis for precise lesion identification and characterization.
- Personalized stent selection and sizing optimized by AI algorithms based on patient-specific anatomical data and disease progression.
- Improved procedural guidance and navigation using AI-driven real-time imaging for accurate stent deployment and reduced complications.
- Predictive analytics for anticipating potential post-procedure complications such as migration, re-obstruction, or infection.
- Streamlined research and development for novel stent materials and designs through AI-driven simulation and data analysis.
- Automated monitoring of stent performance and patient recovery via AI-enabled remote sensing technologies.
- Optimization of healthcare resource allocation and operational efficiency in endoscopic units through AI-driven scheduling and inventory management.
DRO & Impact Forces Of Gastrointestinal Stents Market
The Gastrointestinal Stents Market is propelled by a robust set of drivers, primarily the escalating global incidence of gastrointestinal disorders, including various forms of cancer (esophageal, pancreatic, colorectal) and benign conditions like strictures and fistulas. The increasing aging population, which is more susceptible to these chronic conditions, significantly contributes to the demand for effective palliative and therapeutic interventions. Furthermore, the growing preference for minimally invasive endoscopic procedures over traditional open surgery, driven by benefits such as reduced recovery times, lower complication rates, and shorter hospital stays, acts as a strong market accelerator. Technological advancements in stent design, materials (e.g., nitinol alloys, biodegradable polymers), and delivery systems continually improve efficacy and expand clinical applications, making stents a more viable option for a broader patient demographic. Public and physician awareness campaigns regarding early diagnosis and treatment options for GI conditions also play a crucial role in market expansion.
Conversely, the market faces several significant restraints. The high cost associated with advanced gastrointestinal stents and the endoscopic procedures required for their placement can be a barrier to adoption, particularly in developing regions with limited healthcare budgets or inadequate insurance coverage. The risk of various complications post-stent insertion, such as stent migration, re-obstruction (due to tissue ingrowth or overgrowth), perforation, and infection, remains a concern for both clinicians and patients, necessitating careful patient selection and post-procedure monitoring. Moreover, the stringent regulatory approval processes for new medical devices, including GI stents, in major markets like North America and Europe, can lead to extended development timelines and increased R&D costs, potentially stifling innovation and delaying market entry for novel products. Ethical considerations surrounding long-term device safety and efficacy also contribute to regulatory scrutiny.
Despite these challenges, substantial opportunities exist within the market. The development and commercialization of innovative biodegradable and drug-eluting stents represent a significant growth avenue, promising improved clinical outcomes by reducing the need for stent removal and preventing restenosis. Emerging markets, particularly in Asia Pacific, Latin America, and the Middle East and Africa, offer untapped potential due to their large patient populations, improving healthcare infrastructure, and increasing healthcare spending. Strategic partnerships with local distributors and healthcare providers are key to unlocking these regional opportunities. Furthermore, the integration of advanced imaging technologies, artificial intelligence, and robotic assistance into endoscopic procedures is set to enhance the precision and safety of stent placement, creating new niches for technological differentiation and competitive advantage. The expansion of indications for stent use into new therapeutic areas also offers promising growth prospects. The collective impact forces of healthcare expenditure, evolving regulatory landscape, and increasing patient awareness will continue to shape the trajectory of this dynamic market.
Segmentation Analysis
The Gastrointestinal Stents Market is comprehensively segmented across various parameters, allowing for a detailed analysis of market dynamics, competitive landscapes, and growth opportunities. These segmentation strategies include classification by product type, the material composition of the stent, its specific application in treating either malignant or benign obstructions, and the end-user facilities where these procedures are primarily conducted. This multi-faceted approach provides granular insights into consumer preferences, technological advancements, and regional adoption rates, enabling stakeholders to identify high-growth segments and tailor their strategies accordingly. The diversity in product offerings and applications reflects the broad spectrum of gastrointestinal conditions requiring stent intervention.
- Product Type:
- Esophageal Stents: Used for obstructions in the esophagus, typically due to cancer or strictures.
- Biliary Stents: Employed in the bile ducts for malignant or benign obstructions causing jaundice.
- Colonic Stents: Applied in the colon for obstructions, often as a bridge to surgery or for palliative care.
- Duodenal Stents: Used to relieve obstructions in the duodenum, commonly caused by pancreatic cancer or strictures.
- Pancreatic Stents: Inserted into the pancreatic duct for strictures or leaks.
- Material:
- Self-Expandable Metallic Stents (SEMS): Predominantly made from nitinol, known for high radial force and long-term patency.
- Plastic Stents: Typically made from polyethylene or polyurethane, often used for temporary applications.
- Biodegradable Stents: Designed to gradually dissolve, eliminating the need for retrieval procedures.
- Application:
- Malignant Obstructions: Stents used to relieve obstructions caused by cancerous growths (e.g., esophageal cancer, pancreatic cancer).
- Benign Obstructions: Stents used for non-cancerous strictures, fistulas, or other benign conditions.
- End User:
- Hospitals: The largest end-user segment due to advanced infrastructure and high patient volume.
- Ambulatory Surgical Centers (ASCs): Growing segment due to increasing preference for outpatient procedures.
- Specialty Clinics: Facilities focusing on specific gastroenterological or oncological treatments.
Value Chain Analysis For Gastrointestinal Stents Market
The value chain for the Gastrointestinal Stents Market is a complex network involving multiple stages, from raw material sourcing to final patient use, each contributing to the overall product delivery and market value. The upstream segment of the value chain is dominated by specialized suppliers of high-grade raw materials such as nitinol alloys, stainless steel, and various medical-grade polymers like polyethylene, silicone, and biodegradable compounds (e.g., PLA, PCL). These suppliers often possess advanced manufacturing capabilities to produce components tailored to the specific requirements of medical device manufacturers, ensuring biocompatibility, mechanical integrity, and durability. Research and development activities, including clinical trials and regulatory submissions, are also critical upstream elements, driving innovation and securing market access for new stent technologies. Component manufacturers, specializing in braiding, laser cutting, and surface treatment, form another crucial part of the upstream segment, transforming raw materials into semi-finished stent parts.
Moving downstream, the value chain focuses on the manufacturing, assembly, and sterilization of the final stent products. Medical device companies typically integrate these components, conducting rigorous quality control and testing processes to ensure compliance with international medical device standards. Once manufactured, the distribution channels play a pivotal role in delivering these specialized devices to healthcare providers globally. Direct distribution involves sales teams from major manufacturers engaging directly with large hospitals and academic medical centers, offering product training and technical support. This approach allows for greater control over sales and customer relationships, particularly for high-value or complex products. Indirect distribution, on the other hand, involves leveraging a network of third-party distributors, wholesalers, and medical supply companies to reach a broader base of smaller hospitals, ambulatory surgical centers, and specialty clinics across diverse geographical regions. These distributors often have established logistics networks and local market knowledge, facilitating wider market penetration.
The final stage of the value chain involves the end-users, primarily hospitals, ambulatory surgical centers, and specialty clinics, where gastroenterologists, oncologists, interventional radiologists, and surgeons deploy the stents in patients. Post-sales support, including device monitoring, adverse event reporting, and continuous medical education, further enhances the product's value proposition. The efficiency and effectiveness of this entire value chain are critical for market success, impacting product availability, cost-effectiveness, and ultimately, patient outcomes. Optimizing each stage, from sourcing to patient care, is essential for maintaining competitive advantage and driving innovation in the gastrointestinal stents market.
Gastrointestinal Stents Market Potential Customers
The primary end-users and buyers in the Gastrointestinal Stents Market are diverse, encompassing various medical professionals and healthcare institutions that directly or indirectly facilitate the use of these devices for patient care. Gastroenterologists represent a significant customer segment, as they are at the forefront of diagnosing and managing gastrointestinal diseases and regularly perform endoscopic procedures for stent placement. Oncologists also constitute a crucial group, particularly for patients with malignant obstructions caused by various cancers, where stents are vital for palliative symptom relief or as part of a multi-modal treatment strategy. Interventional radiologists, with their expertise in image-guided procedures, are increasingly involved in placing stents in complex cases, especially in the biliary and pancreatic ducts, further expanding the customer base.
Beyond individual specialists, hospital procurement departments are major buyers, responsible for acquiring a consistent supply of gastrointestinal stents for their endoscopy units, oncology departments, and surgical suites. The decision-making process in hospitals often involves committees that evaluate product efficacy, cost-effectiveness, and supplier reliability. Ambulatory Surgical Centers (ASCs) are also emerging as key customers, particularly for elective or less complex stent placement procedures, driven by their focus on efficiency and outpatient care models. Specialty clinics that focus on gastroenterology or oncology also represent a specific customer segment, seeking specialized stents and complementary devices for their patient populations. Ultimately, the indirect beneficiaries and core focus of the market are patients suffering from gastrointestinal obstructions, whose needs drive the demand and innovation within this market, making patient-centric solutions paramount for all stakeholders.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | $475 Million |
| Market Forecast in 2032 | $755 Million |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Boston Scientific Corporation, Cook Medical, Olympus Corporation, ConMed Corporation, Medtronic plc, Gore Medical, Merit Medical Systems, Inc., Hobbs Medical, B. Braun Melsungen AG, Teleflex Incorporated, ELLA CS, S.R.O., Micro-Tech Endoscopy, Taewoong Medical, C. R. Bard (BD), ENDO-FLEX GmbH, Sumitomo Bakelite Co., Ltd., Leufen Medical GmbH, Novatech SA, Stentec, Q3 Medical Devices |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Gastrointestinal Stents Market Key Technology Landscape
The Gastrointestinal Stents Market is characterized by a dynamic and evolving technology landscape, continuously striving for improved patient outcomes, enhanced procedural safety, and expanded clinical utility. A cornerstone of current stent technology involves the use of advanced materials, prominently nitinol alloys for self-expandable metallic stents (SEMS). Nitinol's superelasticity and shape memory properties allow stents to be compressed for delivery through narrow endoscopic channels and then expand to their pre-set diameter once deployed, providing excellent radial force and flexibility to conform to anatomical curves while resisting compression. Stainless steel is also utilized, though less frequently than nitinol for self-expanding designs, often finding application in balloon-expandable stents.
Beyond metallic stents, the technology landscape includes plastic stents, typically made from polyethylene or polyurethane, which are often used for temporary drainage or dilation. However, the most significant area of innovation lies in the development of biodegradable stents, which are engineered from absorbable polymers such as poly-L-lactic acid (PLLA), polycaprolactone (PCL), or polydioxanone (PDO). These stents offer the advantage of gradual dissolution over time, negating the need for subsequent endoscopic removal procedures and potentially reducing long-term complications associated with permanent implants. This technology addresses a critical unmet need for temporary stenting applications where a permanent implant is undesirable.
Further technological advancements include the incorporation of drug-eluting coatings onto stent surfaces to prevent tissue ingrowth and restenosis, particularly in benign strictures or certain malignant conditions. These coatings often release antiproliferative or anti-inflammatory agents to modulate cellular response at the stent site. Anti-reflux mechanisms, such as specialized valve designs or asymmetric stent flares, are also being integrated into esophageal stents to minimize gastroesophageal reflux. The development of radiopaque markers on stents enhances visibility under fluoroscopy, facilitating precise placement and subsequent monitoring. Moreover, improvements in endoscopic delivery systems, including smaller diameters, greater flexibility, and enhanced maneuverability, are making stent placement procedures safer and more accessible. Overall, the technological trajectory of GI stents is focused on smart materials, localized drug delivery, and improved deployment accuracy, reflecting a commitment to innovation-driven patient care.
Regional Highlights
- North America: This region holds a significant share of the gastrointestinal stents market, driven by a high prevalence of GI cancers, well-established healthcare infrastructure, advanced diagnostic capabilities, and widespread adoption of minimally invasive endoscopic procedures. Favorable reimbursement policies, substantial healthcare expenditure, and the presence of key market players further solidify its leading position. The United States, in particular, is a major contributor to the regional market due to its large patient pool and continuous technological advancements in medical devices.
- Europe: Europe represents another substantial market for GI stents, characterized by robust healthcare systems, a growing geriatric population, and increasing awareness regarding early diagnosis and treatment of GI disorders. Countries like Germany, France, and the United Kingdom are key contributors, benefiting from significant R&D investments, advanced medical facilities, and a strong preference for modern treatment modalities. Regulatory frameworks are mature, fostering innovation while ensuring patient safety.
- Asia Pacific (APAC): The Asia Pacific region is projected to be the fastest-growing market for gastrointestinal stents. This growth is primarily fueled by a large and rapidly aging population, increasing disposable incomes, improving healthcare infrastructure, and a rising prevalence of chronic gastrointestinal diseases. Countries such as China, India, and Japan are at the forefront, witnessing substantial investments in healthcare, expanding access to advanced medical treatments, and a growing adoption of cutting-edge endoscopic technologies. The region also offers significant opportunities for market penetration due to its large untapped patient base.
- Latin America: This region exhibits steady growth in the GI stents market, driven by increasing healthcare expenditure, improving access to medical facilities, and a rising awareness of advanced treatment options. Economic development and government initiatives to enhance public health infrastructure contribute to market expansion, though adoption rates can vary significantly between countries within the region.
- Middle East and Africa (MEA): The MEA region is witnessing gradual growth in the gastrointestinal stents market. This growth is supported by increasing investments in healthcare infrastructure, growing medical tourism, and a rising prevalence of GI disorders in urban centers. Challenges include varying levels of healthcare access and economic disparities across countries, but opportunities exist as healthcare systems continue to develop and modernize.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Gastrointestinal Stents Market.- Boston Scientific Corporation
- Cook Medical
- Olympus Corporation
- ConMed Corporation
- Medtronic plc
- Gore Medical
- Merit Medical Systems, Inc.
- Hobbs Medical
- B. Braun Melsungen AG
- Teleflex Incorporated
- ELLA CS, S.R.O.
- Micro-Tech Endoscopy
- Taewoong Medical
- C. R. Bard (BD)
- ENDO-FLEX GmbH
- Sumitomo Bakelite Co., Ltd.
- Leufen Medical GmbH
- Novatech SA
- Stentec
- Q3 Medical Devices
Frequently Asked Questions
What are gastrointestinal stents used for?
Gastrointestinal stents are specialized medical devices primarily used to relieve obstructions in various parts of the digestive tract, including the esophagus, bile ducts, duodenum, and colon. These obstructions can be caused by malignant tumors, benign strictures, or other conditions, impeding the natural flow of food, fluids, or bile.
What types of GI stents are available?
The market offers several types of GI stents, broadly categorized by material and location. Key types include Self-Expandable Metallic Stents (SEMS) made from nitinol, plastic stents typically made from polyethylene, and emerging biodegradable stents. They are designed for specific anatomical locations such as esophageal, biliary, duodenal, colonic, and pancreatic applications.
What are the main advantages of using GI stents?
The primary advantages of gastrointestinal stents include their minimally invasive placement via endoscopy, which leads to quicker recovery times, reduced pain, and fewer complications compared to surgical alternatives. They provide effective symptomatic relief, such as improving swallowing (dysphagia) or relieving jaundice, significantly enhancing a patient's quality of life, especially in palliative care scenarios.
What are the potential risks associated with GI stent placement?
While generally safe, GI stent placement carries potential risks including stent migration, re-obstruction due to tissue ingrowth or overgrowth, pain, perforation of the GI tract, and infection. Careful patient selection, precise placement, and post-procedure monitoring are crucial to minimize these complications and ensure optimal outcomes.
How is AI impacting the Gastrointestinal Stents Market?
Artificial intelligence is increasingly impacting the GI stents market by enhancing diagnostic accuracy through advanced image analysis, enabling personalized stent selection based on patient-specific data, and improving procedural guidance during deployment. AI also assists in predictive analytics for complications and accelerates the research and development of novel stent designs and materials, leading to more efficient and effective patient care.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
