Hepatitis E Diagnostic Tests Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429299 | Date : Oct, 2025 | Pages : 255 | Region : Global | Publisher : MRU
Hepatitis E Diagnostic Tests Market Size
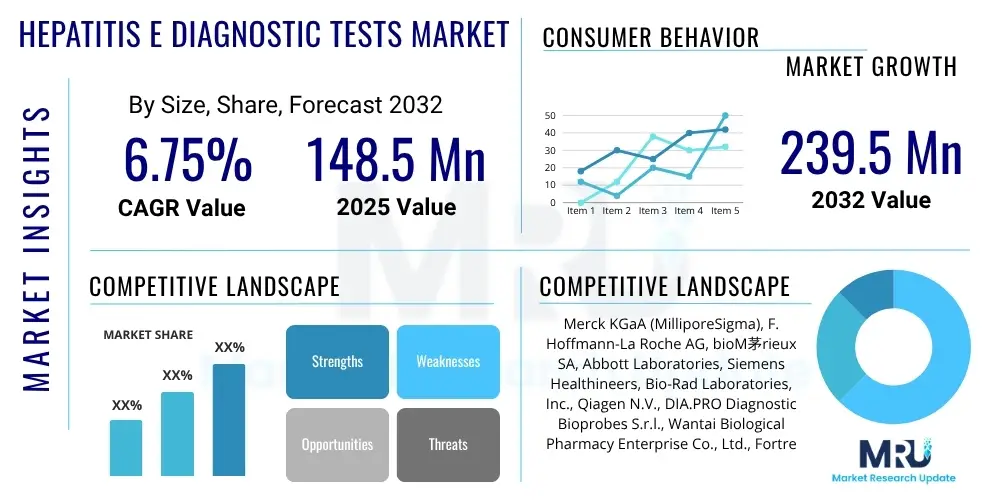
The Hepatitis E Diagnostic Tests Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.75% between 2025 and 2032. The market is estimated at USD 148.5 million in 2025 and is projected to reach USD 239.5 million by the end of the forecast period in 2032.
Hepatitis E Diagnostic Tests Market introduction
The Hepatitis E Diagnostic Tests Market encompasses the global landscape of solutions utilized for the detection and diagnosis of Hepatitis E Virus (HEV) infection. Hepatitis E is a significant public health concern, particularly in regions with poor sanitation and contaminated water sources, manifesting as acute or, less commonly, chronic hepatitis. The primary product offerings within this market include various immunoassay kits, such as ELISA (Enzyme-Linked Immunosorbent Assay) for detecting anti-HEV antibodies (IgM and IgG), and molecular diagnostic tests, primarily RT-PCR (Reverse Transcription Polymerase Chain Reaction) assays, which identify HEV RNA. These tests are crucial for accurate and timely diagnosis, distinguishing HEV from other forms of viral hepatitis, especially in immunocompromised individuals or pregnant women where the infection can be severe.
Major applications of these diagnostic tests span across clinical diagnosis, epidemiological surveillance, blood donor screening, and research. In clinical settings, they are indispensable for confirming acute HEV infections and monitoring disease progression. For public health, these tests enable the identification of outbreaks and assessment of disease prevalence, informing preventative strategies. The benefits of advanced Hepatitis E diagnostic tests include enhanced accuracy, reduced turnaround time, and improved sensitivity, leading to better patient outcomes and public health management. These advantages are particularly vital in developing regions where HEV is endemic, allowing for earlier intervention and preventing severe complications or widespread transmission.
The market is primarily driven by the increasing global prevalence of Hepatitis E, heightened awareness among healthcare professionals and the public, and ongoing advancements in diagnostic technologies that offer more rapid and reliable detection methods. Additionally, growing investments in healthcare infrastructure, particularly in emerging economies, and the increasing demand for advanced diagnostic solutions for infectious diseases contribute significantly to market expansion. The continuous efforts in research and development to introduce more accessible and cost-effective testing platforms further fuel the market's growth trajectory.
Hepatitis E Diagnostic Tests Market Executive Summary
The Hepatitis E Diagnostic Tests market is currently experiencing robust growth, driven by an escalating global disease burden and a strong emphasis on early and accurate diagnosis to mitigate health complications. Business trends indicate a shift towards rapid, point-of-care testing solutions and molecular diagnostics, reflecting the demand for faster turnaround times and higher specificity. Companies are focusing on strategic collaborations, mergers, and acquisitions to expand their product portfolios and geographical reach, particularly in high-prevalence areas. There is also a notable trend towards developing multi-parameter diagnostic platforms capable of detecting various infectious agents, including HEV, within a single test, streamlining diagnostic workflows and enhancing efficiency for healthcare providers. This competitive landscape fosters innovation and encourages the adoption of sophisticated diagnostic technologies.
Regionally, the market exhibits varied dynamics. Asia Pacific and Africa are identified as high-growth regions due to the endemic nature of Hepatitis E, large populations, and improving healthcare access, albeit with persistent challenges in sanitation and clean water. North America and Europe, characterized by advanced healthcare infrastructures and higher diagnostic expenditure, show steady growth driven by the need for blood donor screening and the diagnosis of imported cases or atypical presentations. Latin America is also emerging as a significant market, influenced by improving diagnostic capabilities and increasing public health initiatives. Each region presents unique opportunities and challenges, requiring tailored market strategies to address specific epidemiological patterns and healthcare system capabilities.
Segmentation trends highlight the dominance of immunoassay-based tests, particularly ELISA for antibody detection, owing to their cost-effectiveness and ease of use, making them accessible for broader screening efforts. However, molecular diagnostics, like RT-PCR, are gaining traction due to their superior sensitivity and ability to detect active viral replication, crucial for early diagnosis and monitoring. End-user segments such as hospitals and diagnostic laboratories continue to be the largest consumers, driven by their central role in patient care and routine testing. The research and academic institutes segment is also expanding, propelled by ongoing studies into HEV pathogenesis, vaccine development, and diagnostic innovation. The consistent evolution across these segments underscores a dynamic market poised for sustained expansion as diagnostic technologies become more refined and widely adopted.
AI Impact Analysis on Hepatitis E Diagnostic Tests Market
Common user questions regarding AI's impact on the Hepatitis E Diagnostic Tests Market often revolve around how artificial intelligence can enhance diagnostic accuracy, speed up test results, reduce costs, and contribute to personalized medicine approaches for HEV. Users are keen to understand if AI can improve the interpretation of complex diagnostic data, particularly from molecular assays or imaging techniques, to provide more definitive diagnoses. Concerns also surface about the integration challenges of AI into existing lab infrastructure, data privacy issues, and the need for rigorous validation of AI-powered diagnostic tools to ensure reliability and regulatory compliance. Expectations are high that AI could transform the market by enabling more efficient outbreak prediction, improving surveillance systems, and ultimately leading to more proactive public health interventions against Hepatitis E.
- AI can significantly enhance diagnostic accuracy by analyzing complex patterns in immunoassay or molecular data, identifying subtle markers potentially missed by human interpretation, thereby reducing false positives and negatives.
- Integration of AI in automated diagnostic platforms can accelerate the processing and interpretation of Hepatitis E test results, leading to quicker patient management decisions and improved clinical workflows.
- AI-powered tools can optimize laboratory operations, predicting reagent needs, managing sample queues, and automating quality control, potentially leading to cost efficiencies in diagnostic testing.
- Machine learning algorithms can be trained on large datasets of patient symptoms, risk factors, and test results to identify individuals at higher risk of HEV infection, enabling targeted screening programs.
- AI can assist in real-time epidemiological surveillance by processing vast amounts of data from diagnostic labs and public health records, facilitating early detection of HEV outbreaks and informing rapid response strategies.
- Development of AI-driven image analysis for liver biopsies or other imaging modalities could aid in assessing the severity of HEV-induced liver damage and monitoring treatment efficacy.
- AI can contribute to the development of novel diagnostic biomarkers for Hepatitis E by identifying new correlational patterns within genomic or proteomic data, leading to more sensitive and specific tests.
- Predictive analytics powered by AI could forecast regional HEV prevalence and identify environmental factors contributing to transmission, allowing for proactive public health interventions and resource allocation.
DRO & Impact Forces Of Hepatitis E Diagnostic Tests Market
The Hepatitis E Diagnostic Tests market is propelled by several key drivers, including the increasing global incidence and prevalence of HEV infections, particularly in endemic regions, which necessitates more widespread and accurate diagnostic capabilities. Significant advancements in diagnostic technologies, such as the development of highly sensitive and specific molecular assays and rapid immunoassay kits, also play a crucial role in expanding market accessibility and efficacy. Furthermore, growing public health initiatives and awareness campaigns aimed at controlling infectious diseases, coupled with enhanced surveillance programs for blood safety and disease outbreaks, amplify the demand for reliable HEV diagnostic tools. The rising healthcare expenditure and improving diagnostic infrastructure in developing economies further contribute to market expansion by enabling greater access to advanced testing facilities and promoting early diagnosis.
However, the market faces notable restraints that could temper its growth. The relatively high cost of advanced diagnostic tests, particularly molecular assays, can pose a barrier to adoption in low-income settings or regions with limited healthcare budgets. Lack of widespread awareness regarding Hepatitis E symptoms and transmission routes in some populations also leads to underdiagnosis and underreporting, thereby hindering market potential. Additionally, challenges in sample collection, transportation, and storage in remote areas, along with the need for skilled personnel to operate sophisticated diagnostic equipment, represent operational hurdles. Regulatory complexities and varied reimbursement policies across different geographies can also create inconsistencies in market access and product commercialization.
Despite these restraints, significant opportunities exist for market players. The development of cost-effective, point-of-care (POC) diagnostic devices that can provide rapid results without extensive laboratory infrastructure presents a major opportunity, especially for resource-limited settings and outbreak response. Expanding into untapped emerging markets, where HEV prevalence is high but diagnostic penetration is low, offers substantial growth potential. Moreover, increasing research and development activities focused on developing multiplex assays that can detect multiple hepatitis viruses simultaneously could enhance diagnostic efficiency and convenience. Strategic partnerships between diagnostic companies and public health organizations to facilitate large-scale screening and surveillance programs also represent a promising avenue for market expansion, ensuring broader access to testing and improving global health outcomes against Hepatitis E.
Segmentation Analysis
The Hepatitis E Diagnostic Tests market is comprehensively segmented based on various critical parameters including test type, sample type, end-user, and geographic region. This segmentation provides a granular view of the market dynamics, allowing for a detailed understanding of consumer preferences, technological adoption trends, and regional specificities. The diverse range of diagnostic approaches and applications caters to a broad spectrum of clinical and public health needs, from acute infection detection to epidemiological studies and blood bank screening, ensuring a robust and adaptable market landscape. Each segment plays a vital role in addressing the multifaceted challenges posed by Hepatitis E globally, driving innovation and expanding access to effective diagnostic solutions.
- By Test Type
- ELISA (Enzyme-Linked Immunosorbent Assay)
- IgM ELISA
- IgG ELISA
- RT-PCR (Reverse Transcription Polymerase Chain Reaction)
- Real-time RT-PCR
- Nested RT-PCR
- Rapid Tests
- Other Tests (e.g., Immunochromatographic Assay, Western Blot)
- ELISA (Enzyme-Linked Immunosorbent Assay)
- By Sample Type
- Blood
- Serum/Plasma
- Stool
- Urine
- Other Biological Fluids
- By End-User
- Hospitals
- Diagnostic Laboratories
- Blood Banks
- Research & Academic Institutes
- Public Health Agencies
- Reference Laboratories
- By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Value Chain Analysis For Hepatitis E Diagnostic Tests Market
The value chain for the Hepatitis E Diagnostic Tests market initiates with upstream activities involving the research and development of novel diagnostic technologies and the procurement of essential raw materials. This includes the acquisition of reagents, enzymes, antibodies, antigens, and other biochemical components necessary for the formulation of test kits. Key players in this stage are specialized biotechnology companies and chemical suppliers that provide high-quality, purified biological and chemical ingredients. The stringent quality control measures at this stage are paramount to ensuring the reliability and accuracy of the final diagnostic products. Manufacturers invest significantly in R&D to enhance test sensitivity, specificity, and user-friendliness, continually improving assay performance and reducing turnaround times.
Following the upstream phase, the manufacturing process involves the assembly, formulation, and packaging of various diagnostic kits, such as ELISA plates, RT-PCR reagents, and rapid test devices. This stage incorporates sophisticated production techniques and quality assurance protocols to ensure consistency and compliance with global regulatory standards, including FDA, CE-IVD, and other regional certifications. Downstream activities encompass the distribution and sales of these diagnostic products to a diverse range of end-users. The distribution channel is multifaceted, comprising direct sales forces for large clients like major hospitals and reference laboratories, and indirect channels involving a network of distributors, wholesalers, and third-party logistics providers who facilitate market penetration in broader geographical areas, including remote and underserved regions.
The direct sales approach enables manufacturers to build strong relationships with key customers, providing specialized support, training, and technical services, which is particularly beneficial for complex molecular diagnostic platforms. Indirect channels, on the other hand, are crucial for reaching a wider customer base and optimizing logistics, especially in regions with fragmented healthcare systems. Both channels are essential for market reach and customer service. Furthermore, post-sales support, including technical troubleshooting, maintenance, and educational programs for laboratory personnel, adds significant value, ensuring optimal utilization of diagnostic tests and maintaining customer satisfaction. The efficiency and reliability of this entire value chain are critical for the effective and timely diagnosis of Hepatitis E, contributing to improved public health outcomes globally.
Hepatitis E Diagnostic Tests Market Potential Customers
The primary potential customers for Hepatitis E diagnostic tests are diverse and include a wide array of healthcare institutions and public health entities that play a critical role in disease diagnosis, surveillance, and management. Hospitals, particularly those with infectious disease departments, gastroenterology units, and critical care facilities, represent a significant end-user segment. They require these tests for routine patient diagnostics, differential diagnosis of acute hepatitis, and managing complications in specific patient populations such as pregnant women and immunocompromised individuals, where HEV can lead to severe clinical outcomes. The need for rapid and accurate results in these settings is paramount for effective patient care and preventing nosocomial transmission.
Diagnostic laboratories, encompassing both independent reference laboratories and those affiliated with hospitals, constitute another major segment of potential customers. These laboratories perform a high volume of HEV tests, often processing samples referred from various clinical settings. Their demand is driven by the necessity for advanced testing platforms, high throughput capabilities, and adherence to stringent quality standards for accurate detection of anti-HEV antibodies and viral RNA. Blood banks also represent a crucial customer segment, as mandatory screening of blood donations for infectious agents, including HEV, is critical to ensure the safety of the blood supply and prevent transfusion-transmitted infections, especially in regions with high HEV prevalence. This proactive screening protects recipients from potential viral transmission.
Furthermore, research and academic institutes frequently utilize Hepatitis E diagnostic tests for epidemiological studies, pathogen surveillance, vaccine development, and understanding the pathogenesis of the virus. Their demand is often for specialized reagents, highly sensitive assays, and customized testing solutions for research purposes. Public health agencies and government organizations are also significant potential customers, particularly for large-scale population screening programs, outbreak investigations, and monitoring disease trends to implement effective control and prevention strategies. The procurement by these entities is vital for global health security and informs public health policies. Each of these customer groups exhibits unique requirements and purchasing patterns, necessitating tailored marketing and product development strategies from diagnostic test providers.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 148.5 Million |
| Market Forecast in 2032 | USD 239.5 Million |
| Growth Rate | 6.75% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces | |
| Segments Covered | |
| Key Companies Covered | Merck KGaA (MilliporeSigma), F. Hoffmann-La Roche AG, bioM茅rieux SA, Abbott Laboratories, Siemens Healthineers, Bio-Rad Laboratories, Inc., Qiagen N.V., DIA.PRO Diagnostic Bioprobes S.r.l., Wantai Biological Pharmacy Enterprise Co., Ltd., Fortress Diagnostics, DRG International, Inc., Beijing Luzhu Biotechnology Co., Ltd., Mikrogen GmbH, NovaTec Immundiagnostica GmbH, Sysmex Corporation, CTK Biotech, Inc., OraSure Technologies, Inc., Euroimmun AG, Grifols S.A., Analytik Jena AG. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Hepatitis E Diagnostic Tests Market Key Technology Landscape
The Hepatitis E Diagnostic Tests market is characterized by a dynamic and evolving technology landscape, primarily driven by the continuous demand for enhanced sensitivity, specificity, speed, and cost-effectiveness in detection. Immunoassays, predominantly Enzyme-Linked Immunosorbent Assay (ELISA), remain a foundational technology for screening and diagnosis due to their relatively low cost and high throughput capabilities. ELISA kits are widely used for detecting both IgM antibodies, indicating acute or recent infection, and IgG antibodies, signaling past exposure or chronic infection. Advancements in ELISA technology focus on improving assay design, antigen presentation, and conjugate systems to enhance performance, reduce cross-reactivity with other viral antibodies, and achieve higher sensitivity for early detection, making them a staple in routine diagnostics and epidemiological studies.
Molecular diagnostic techniques, particularly Reverse Transcription Polymerase Chain Reaction (RT-PCR), represent the gold standard for directly detecting the Hepatitis E viral RNA. Real-time RT-PCR assays are crucial for confirming active infections, particularly in immunocompromised patients, pregnant women, or during outbreak investigations where early and precise detection is critical. These technologies offer superior sensitivity and specificity, allowing for quantification of viral load, which is valuable for monitoring disease progression and treatment efficacy. Innovations in RT-PCR involve multiplexing capabilities, enabling the simultaneous detection of multiple pathogens or different HEV genotypes, thereby increasing efficiency and reducing turnaround time. The development of automated nucleic acid extraction and amplification systems further streamlines the workflow, making these advanced tests more accessible for high-volume laboratories.
Emerging technologies also significantly contribute to the market's technological landscape. Rapid diagnostic tests (RDTs), based on immunochromatography, offer quick, user-friendly, and portable solutions for point-of-care (POC) testing, particularly beneficial in resource-limited settings or for initial screening in remote areas. While typically less sensitive than laboratory-based methods, their speed and ease of use make them valuable for immediate triage. Furthermore, advancements in bioinformatics and microfluidics are paving the way for next-generation sequencing (NGS) and lab-on-a-chip technologies, which promise even greater diagnostic precision, capacity for comprehensive genetic analysis of the virus, and integration into compact, automated platforms. These innovations collectively drive the market towards more efficient, accurate, and accessible Hepatitis E diagnostic solutions, addressing a broader range of clinical and public health needs globally.
Regional Highlights
- Asia Pacific: This region accounts for the highest burden of Hepatitis E, particularly in countries like India, China, and Southeast Asian nations, due to factors such as poor sanitation and contaminated water sources. The market here is driven by the urgent need for widespread diagnostic testing, increasing public health initiatives, and improving healthcare infrastructure. Growth is significant, fueled by large populations and growing awareness, alongside government investments in infectious disease control.
- Africa: Similar to Asia Pacific, Africa faces a substantial HEV burden with frequent outbreaks, especially in sub-Saharan regions. The market is characterized by a high demand for cost-effective and rapid diagnostic solutions suitable for remote and resource-limited settings. International aid and collaborations with NGOs play a crucial role in enhancing diagnostic capabilities and expanding access to testing.
- North America: In North America, Hepatitis E is less prevalent but typically associated with travel to endemic regions or zoonotic transmission (e.g., from undercooked pork). The market is driven by sophisticated blood donor screening programs, advanced diagnostic technologies (including molecular testing), and the diagnosis of atypical clinical presentations. High healthcare expenditure and a robust research ecosystem contribute to the adoption of cutting-edge diagnostic tools.
- Europe: European countries experience sporadic cases, often related to travel or indigenous zoonotic transmission. The market is characterized by stringent blood safety regulations, a strong focus on epidemiological surveillance, and a high demand for highly accurate diagnostic tests. There is a growing emphasis on differentiating HEV from other liver diseases, boosting the demand for specific and sensitive assays.
- Latin America: The prevalence of Hepatitis E in Latin America is heterogeneous but generally considered intermediate. The market is driven by efforts to improve public health infrastructure, increasing awareness campaigns, and the adoption of modern diagnostic techniques. Economic development and investments in healthcare are gradually expanding access to testing and surveillance programs across the region.
- Middle East & Africa (MEA): This region presents a mixed landscape, with some areas experiencing higher HEV endemicity than others. Challenges related to healthcare access and infrastructure are present, but growing healthcare investments and rising awareness are pushing market growth. The focus is on implementing effective surveillance and diagnostic strategies to manage outbreaks and reduce disease transmission, often supported by international health organizations.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Hepatitis E Diagnostic Tests Market.- Merck KGaA (MilliporeSigma)
- F. Hoffmann-La Roche AG
- bioM茅rieux SA
- Abbott Laboratories
- Siemens Healthineers
- Bio-Rad Laboratories, Inc.
- Qiagen N.V.
- DIA.PRO Diagnostic Bioprobes S.r.l.
- Wantai Biological Pharmacy Enterprise Co., Ltd.
- Fortress Diagnostics
- DRG International, Inc.
- Beijing Luzhu Biotechnology Co., Ltd.
- Mikrogen GmbH
- NovaTec Immundiagnostica GmbH
- Sysmex Corporation
- CTK Biotech, Inc.
- OraSure Technologies, Inc.
- Euroimmun AG
- Grifols S.A.
- Analytik Jena AG
Frequently Asked Questions
Analyze common user questions about the Hepatitis E Diagnostic Tests market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is Hepatitis E and why are diagnostic tests important?
Hepatitis E is a liver infection caused by the Hepatitis E Virus (HEV), often transmitted through contaminated water or food. Diagnostic tests are crucial for accurate and timely identification of HEV infection, enabling proper patient management, preventing severe complications (especially in vulnerable populations), and aiding public health surveillance to control outbreaks.
What are the primary types of Hepatitis E diagnostic tests available?
The main types include Immunoassay-based tests, such as ELISA (Enzyme-Linked Immunosorbent Assay), which detect anti-HEV antibodies (IgM for acute infection, IgG for past exposure), and Molecular tests like RT-PCR (Reverse Transcription Polymerase Chain Reaction), which directly detect HEV RNA, confirming active viral presence.
How is the Hepatitis E Diagnostic Tests market expected to grow?
The market is projected for significant growth, driven by increasing global prevalence of HEV, continuous advancements in diagnostic technologies, rising awareness, and expanding healthcare infrastructure in endemic regions. It is expected to grow at a CAGR of 6.75% from 2025 to 2032.
What are the key challenges in the Hepatitis E Diagnostic Tests market?
Key challenges include the high cost of advanced molecular diagnostic tests, limited awareness of HEV in certain populations leading to underdiagnosis, and operational difficulties such as inadequate infrastructure for testing in remote areas and varied regulatory landscapes.
How do Hepatitis E diagnostic tests contribute to blood safety?
HEV diagnostic tests are essential for screening blood donations, particularly in endemic regions, to prevent transfusion-transmitted Hepatitis E. Detecting HEV RNA or antibodies in donated blood ensures the safety of the blood supply, significantly reducing the risk of viral transmission to recipients.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
