In-vitro Transcription Templates Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 431261 | Date : Nov, 2025 | Pages : 255 | Region : Global | Publisher : MRU
In-vitro Transcription Templates Market Size
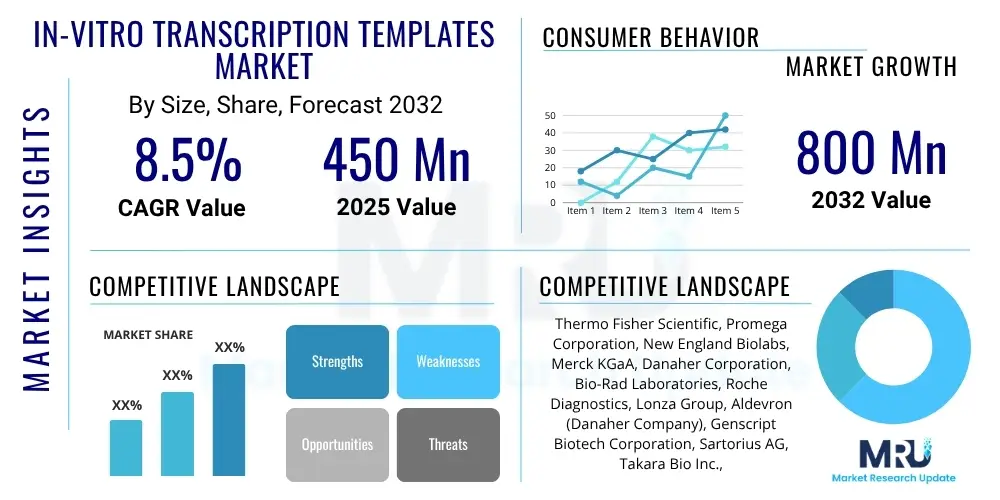
The In-vitro Transcription Templates Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.5% between 2025 and 2032. The market is estimated at USD 450 Million in 2025 and is projected to reach USD 800 Million by the end of the forecast period in 2032.
In-vitro Transcription Templates Market introduction
The In-vitro Transcription Templates Market encompasses the production and supply of DNA sequences specifically designed for in-vitro transcription (IVT), a crucial process for synthesizing RNA molecules outside of living cells. These templates, primarily linearized plasmid DNA or PCR-amplified DNA, serve as the foundational material for generating various RNA types, including messenger RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA), as well as non-coding RNAs for diverse research and therapeutic applications. The increasing demand for high-quality, reliable templates stems from rapid advancements in biotechnology, particularly in areas like mRNA vaccine development, gene therapy, and molecular diagnostics. The market is characterized by ongoing innovation aimed at enhancing template purity, yield, and specific modifications.
The core product in this market involves meticulously prepared DNA templates that contain the desired gene sequence flanked by a promoter recognized by an RNA polymerase (e.g., T7, SP6, or T3). Major applications span across infectious disease research, oncology, rare genetic disorder treatments, and fundamental biological studies. The benefits of utilizing specialized IVT templates include enhanced control over RNA synthesis, the ability to produce large quantities of specific RNA sequences rapidly, and flexibility in incorporating modified nucleotides for improved RNA stability or immunogenicity. Driving factors for market expansion include the significant global investment in mRNA-based therapeutics and vaccines, the burgeoning field of gene editing technologies requiring synthetic RNA components, and the growing adoption of personalized medicine approaches which often rely on custom-designed RNA molecules.
Furthermore, the utility of these templates extends into areas such as drug discovery, where synthetic RNA can be used for target validation and screening, and in synthetic biology for constructing novel biological systems. The templates enable researchers to explore gene function, validate protein expression, and develop advanced diagnostic tools. The market is also propelled by the need for standardized and high-throughput RNA production methods in both academic and industrial settings. This emphasis on efficiency and reproducibility in RNA synthesis workflows underscores the critical role of high-quality in-vitro transcription templates in modern molecular biology and biotechnology.
In-vitro Transcription Templates Market Executive Summary
The In-vitro Transcription Templates Market is poised for substantial growth, primarily driven by the escalating demand for mRNA-based therapies and vaccines, alongside significant advancements in gene editing and diagnostic technologies. Business trends indicate a strong focus on automation and scalability in template production, with companies investing in robust quality control measures and offering custom synthesis services to meet diverse research and therapeutic needs. The shift towards personalized medicine and the rapid response requirements for emerging infectious diseases are accelerating the adoption of IVT template solutions across the biotechnology and pharmaceutical sectors. Strategic collaborations between academic institutions and industry players are also shaping the market landscape, fostering innovation and facilitating the translation of research findings into clinical applications.
Regionally, North America continues to dominate the market due to its robust biotechnology infrastructure, substantial R&D funding, and the presence of numerous key players and leading research universities. Europe also demonstrates significant growth, supported by favorable regulatory frameworks for advanced therapies and a strong pharmaceutical industry base. The Asia Pacific region is emerging as a high-growth market, driven by increasing healthcare expenditure, expanding biotech industries, and rising government support for life science research. Countries like China, India, and South Korea are becoming pivotal centers for R&D and manufacturing of biopharmaceuticals, thereby boosting the demand for IVT templates. Latin America, the Middle East, and Africa are showing nascent but promising growth, primarily through academic collaborations and initial investments in biotechnology.
In terms of segment trends, linearized plasmid DNA templates currently hold a significant market share due to their ease of preparation and established utility, though PCR-amplified DNA templates are gaining traction for their speed and adaptability in certain high-throughput applications. The application segment sees mRNA vaccine development and gene therapy as the leading drivers, with significant investment in these areas. Basic research and diagnostics also represent substantial and consistent demand. End-user segments highlight biotechnology and pharmaceutical companies as the largest consumers, followed by academic and research institutes, and contract research organizations (CROs). The continuous evolution of these segments reflects the dynamic nature of molecular biology and the increasing versatility of RNA-based technologies.
AI Impact Analysis on In-vitro Transcription Templates Market
Common user questions regarding the impact of AI on the In-vitro Transcription Templates Market often revolve around how AI can optimize the design and synthesis of these templates, improve RNA quality and yield, and accelerate the discovery of novel RNA therapeutics. Users are keen to understand if AI can predict the most stable or immunogenic mRNA sequences, automate the laborious process of template preparation, or enhance the overall efficiency of in-vitro transcription reactions. There are also queries about AI's role in analyzing complex experimental data generated from IVT processes and its potential to reduce the cost and time associated with developing new RNA-based products. Key themes emerging from these questions include optimization, automation, predictive analytics, efficiency gains, and data-driven discovery.
AI's influence is anticipated to be transformative, enabling more precise and efficient template design and synthesis workflows. By leveraging machine learning algorithms, researchers can analyze vast datasets of gene sequences, promoter efficiencies, and RNA stability profiles to predict optimal template configurations that lead to higher quality and yield of target RNA. This extends to optimizing the sequence itself for specific purposes, such as enhanced translation efficiency for therapeutic mRNA or improved antigen presentation for vaccines. AI tools can also be integrated into high-throughput screening platforms, significantly accelerating the identification and validation of effective IVT templates, thereby shortening development timelines for new RNA-based therapies and diagnostics.
The application of AI further extends to quality control and process optimization within the IVT workflow. AI-powered analytics can monitor reaction parameters in real-time, identify anomalies, and suggest adjustments to maximize RNA output and purity. This not only reduces experimental variability but also minimizes material waste, contributing to cost-effectiveness. Furthermore, AI can aid in the complex task of designing templates that incorporate modified nucleotides or specific regulatory elements, predicting their impact on RNA function and stability. Overall, AI is expected to streamline the entire lifecycle of in-vitro transcription template development, from initial design to scaled production, ushering in an era of more intelligent and accelerated RNA-based biotechnology.
- Improved template design and sequence optimization for higher RNA yield and stability.
- Automated analysis of high-throughput IVT experimental data, accelerating discovery.
- Predictive modeling for optimal promoter strength and regulatory element integration.
- Enhanced quality control and real-time process monitoring during template synthesis.
- Accelerated identification of novel RNA sequences for therapeutic applications.
- Reduced development time and cost through optimized template generation workflows.
DRO & Impact Forces Of In-vitro Transcription Templates Market
The In-vitro Transcription Templates Market is driven by several powerful forces, prominently including the explosion of research and development in gene therapy and mRNA vaccine technologies. The global success of mRNA vaccines during the COVID-19 pandemic significantly validated and accelerated investment in this platform, creating an unprecedented demand for high-quality IVT templates. Concurrent advancements in synthetic biology, gene editing tools like CRISPR, and the increasing focus on personalized medicine also fuel market expansion. These scientific breakthroughs necessitate reliable and scalable production of custom RNA molecules, directly increasing the consumption of specialized DNA templates. Government funding for biomedical research and a rising awareness of RNA's therapeutic potential further bolster market growth, encouraging both academic and industrial innovation.
However, the market faces notable restraints. The high cost of raw materials, particularly specialized enzymes and nucleotides required for template synthesis and IVT reactions, can be a barrier for smaller research groups or developing nations. Complex and evolving regulatory landscapes, especially for novel gene therapies and mRNA products, introduce significant hurdles and prolong product development timelines, impacting template demand. Technical challenges associated with ensuring the purity, integrity, and scalability of DNA templates, particularly for large-scale therapeutic production, also pose limitations. Variability in template quality from different suppliers can lead to inconsistent IVT results, creating a need for stringent quality control which can be resource-intensive.
Opportunities for market growth are abundant and include the expansion into emerging markets, particularly in Asia Pacific, where healthcare infrastructure is developing rapidly and investment in biotechnology is increasing. The diversification of therapeutic applications beyond vaccines, into areas like cancer immunotherapy, rare genetic disorders, and regenerative medicine, presents vast untapped potential. The development of advanced, high-fidelity RNA polymerases and optimized IVT buffer systems can further enhance template utility and broaden application scope. Strategic collaborations between IVT template providers and therapeutic developers can create symbiotic relationships, accelerating product pipelines. The continuous refinement of template manufacturing processes to reduce costs and improve turnaround times will also unlock new market segments.
The impact forces within this market are multifaceted. Technological shifts, such as the move towards enzymatic DNA synthesis and cell-free DNA production, can dramatically alter the supply chain and production efficiency of IVT templates. Regulatory changes, particularly harmonization efforts for advanced therapies, will either ease or complicate market access. The competitive landscape is intensifying, with both established players and agile startups vying for market share, driving innovation and pricing strategies. Economic factors, including global R&D spending and venture capital investment in biotech, directly influence market dynamics. Lastly, public perception and acceptance of RNA-based medicines will indirectly impact demand for the foundational IVT templates, emphasizing the importance of clear communication and successful clinical outcomes.
Segmentation Analysis
The In-vitro Transcription Templates Market is comprehensively segmented based on various factors, including the type of template used, the primary application area, and the end-user industries. This segmentation provides a granular view of market dynamics, enabling stakeholders to understand demand patterns and identify specific growth opportunities within distinct categories. The types of templates available reflect advancements in DNA synthesis and preparation techniques, each offering unique advantages in terms of purity, yield, and specific use-case suitability. Similarly, the diverse applications highlight the expanding utility of IVT technology across different fields of life science and medicine. Understanding these segments is crucial for strategic planning and resource allocation in this rapidly evolving market.
The primary classifications offer insights into technological preferences and market adoption trends. For instance, while traditional linearized plasmid DNA remains a staple, the increasing adoption of PCR-amplified DNA templates signifies a shift towards faster and more flexible approaches, especially for rapid prototyping or screening. The application-based segmentation clearly demonstrates the therapeutic momentum behind mRNA vaccines and gene therapies, which are significant drivers of current and future market growth. Furthermore, the end-user segmentation helps to pinpoint the key consumer bases, from large pharmaceutical enterprises requiring industrial-scale solutions to academic institutions focused on fundamental research, each with distinct needs and procurement processes.
- By Type
- Linearized Plasmid DNA Templates
- PCR-Amplified DNA Templates
- Others (e.g., Synthetic Oligonucleotides)
- By Application
- mRNA Vaccine Development
- Gene Therapy
- Diagnostics
- Basic Research
- Protein Production
- Drug Discovery and Development
- Others
- By End-User
- Biotechnology and Pharmaceutical Companies
- Academic and Research Institutes
- Contract Research Organizations (CROs)
- Diagnostic Laboratories
- Government Research Organizations
- Others
Value Chain Analysis For In-vitro Transcription Templates Market
The value chain for the In-vitro Transcription Templates Market begins with upstream suppliers providing critical raw materials and reagents essential for DNA template synthesis and purification. This includes manufacturers of high-purity nucleotides, enzymes (such as restriction enzymes, DNA polymerases, ligases), plasmid DNA purification kits, and various chemical reagents. High-quality raw materials are paramount to ensure the integrity and functionality of the downstream IVT templates. These suppliers often specialize in bioprocess materials, adhering to stringent quality standards to meet the demanding requirements of molecular biology research and therapeutic development. The reliability and consistency of these upstream components directly impact the efficiency and success of IVT template production.
Moving downstream, the value chain encompasses the actual manufacturers of in-vitro transcription templates, who synthesize, purify, and characterize the DNA templates according to specific customer requirements. These companies typically offer a range of services from off-the-shelf catalog products to highly customized template synthesis, often incorporating specialized promoters, modified sequences, or specific linearization sites. Once the templates are produced, they are then supplied to end-users. The primary end-users in this market are biotechnology and pharmaceutical companies that utilize these templates for developing mRNA vaccines, gene therapies, and protein production platforms. Academic and research institutes also constitute a significant portion of the downstream market, using templates for fundamental research, functional genomics, and drug target validation. Additionally, contract research organizations (CROs) and diagnostic laboratories leverage these templates for various research and diagnostic assay development activities.
The distribution channels for in-vitro transcription templates are varied, including both direct and indirect models. Direct sales involve manufacturers selling products directly to end-users through their own sales teams and online platforms, allowing for direct communication and specialized support. This model is particularly prevalent for custom orders or large-volume clients. Indirect distribution channels involve partnerships with third-party distributors, life science product suppliers, and e-commerce platforms, which help broaden market reach, especially for standardized catalog products. These distributors often maintain extensive networks and logistics capabilities, facilitating timely delivery to a diverse customer base globally. Effective distribution is crucial for ensuring accessibility and widespread adoption of these specialized research and therapeutic tools. The choice between direct and indirect channels often depends on the company's size, product portfolio, and target customer segments.
In-vitro Transcription Templates Market Potential Customers
The In-vitro Transcription Templates Market serves a diverse range of potential customers, all of whom rely on high-quality DNA templates for the synthesis of various RNA molecules. The primary end-users or buyers of these products are entities involved in advanced biological research, drug discovery, and therapeutic development. These customers are actively engaged in projects that require precise and efficient RNA synthesis, making IVT templates an indispensable component of their experimental and production pipelines. Their needs often span from small-scale research quantities to large-scale, GMP-compliant batches for clinical applications, indicating a demand for flexibility and scalability from suppliers.
Biotechnology and pharmaceutical companies represent a significant customer segment. These organizations are at the forefront of developing novel mRNA vaccines, gene therapies, and other RNA-based therapeutics, where IVT templates are critical for producing the therapeutic RNA. Their requirements often include high purity, specific modifications, and stringent quality control standards to ensure regulatory compliance for clinical trials and commercial manufacturing. Academic and research institutes, including universities, government laboratories, and non-profit organizations, form another substantial customer base. Researchers in these settings utilize IVT templates for fundamental biological studies, functional genomics, protein expression studies, and basic drug discovery research, often requiring diverse templates for a wide array of experimental designs.
Contract Research Organizations (CROs) are also key potential customers, as they provide specialized research services to biotech and pharma companies, often handling various stages of drug development that involve RNA synthesis. Diagnostic laboratories are increasingly using IVT templates for developing and validating molecular diagnostic assays, particularly for infectious diseases and genetic disorders. Lastly, emerging synthetic biology companies and companies focused on agricultural biotechnology or industrial biotechnology may also represent growing customer segments, as they seek to engineer biological systems using synthetic RNA components. The continued expansion of RNA's utility ensures a broad and growing customer base for IVT templates.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 450 Million |
| Market Forecast in 2032 | USD 800 Million |
| Growth Rate | 8.5% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Thermo Fisher Scientific, Promega Corporation, New England Biolabs, Merck KGaA, Danaher Corporation, Bio-Rad Laboratories, Roche Diagnostics, Lonza Group, Aldevron (Danaher Company), Genscript Biotech Corporation, Sartorius AG, Takara Bio Inc., Transcenta Holding Limited, StemRNA Therapeutics Inc., Twist Bioscience Corporation, Creative Biogene, Cytiva (Danaher Company), Sino Biological Inc., OriGene Technologies, VectorBuilder Inc. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
In-vitro Transcription Templates Market Key Technology Landscape
The In-vitro Transcription Templates Market is underpinned by a dynamic technological landscape, focusing on optimizing the design, synthesis, purification, and modification of DNA templates to ensure high-fidelity RNA production. Core technologies include advanced plasmid DNA purification methods, which are crucial for obtaining ultra-pure linearized plasmid DNA templates free of contaminants that could inhibit IVT reactions or affect RNA quality. Techniques such as anion-exchange chromatography and silica-based column purification are widely employed to achieve the high purity levels required, especially for therapeutic applications where even trace impurities can have significant consequences. These purification technologies are continuously being refined to improve yield, reduce processing time, and enhance scalability.
Furthermore, PCR-based amplification technologies play a vital role, particularly for rapid template generation from genomic DNA or cDNA. Optimized PCR protocols and high-fidelity DNA polymerases ensure accurate amplification of the desired gene sequence, which can then be directly used as an IVT template after purification. Advances in synthetic DNA synthesis are also transforming the market, allowing for the de novo creation of custom DNA templates with specific sequence modifications, optimized promoter elements, or the incorporation of non-standard nucleotides. This enables researchers to design templates with unparalleled precision, facilitating the engineering of RNA molecules with desired properties such such as increased stability, enhanced translational efficiency, or reduced immunogenicity.
The integration of automation and high-throughput screening platforms represents another critical technological trend. Automated liquid handling systems and robotic platforms are increasingly used for template preparation, IVT reaction setup, and downstream RNA purification, enabling parallel processing of multiple templates and accelerating research and development cycles. Moreover, advanced analytical techniques such as capillary electrophoresis, mass spectrometry, and next-generation sequencing are essential for comprehensive characterization and quality control of both DNA templates and the resulting RNA transcripts, ensuring their integrity, purity, and functionality. These technological advancements collectively contribute to the efficiency, reliability, and versatility of in-vitro transcription template production, directly impacting the quality and accessibility of RNA-based products.
Regional Highlights
- North America: Dominates the In-vitro Transcription Templates Market due to a robust biotechnology and pharmaceutical industry, significant government and private funding for life science research, and the presence of numerous key market players and leading academic institutions. The region is a hub for mRNA vaccine and gene therapy development, driving high demand for IVT templates. The U.S. leads in R&D investment and commercialization of advanced therapies.
- Europe: Represents a substantial and growing market, propelled by strong research initiatives in genomics, proteomics, and cell and gene therapy. Countries like Germany, the UK, France, and Switzerland have well-established pharmaceutical sectors and supportive regulatory environments for advanced therapeutic medicinal products (ATMPs). Increasing collaborations between academia and industry also contribute to market expansion.
- Asia Pacific (APAC): Emerging as the fastest-growing region, driven by increasing healthcare expenditure, rapid expansion of biotechnology companies, and rising government investments in biomedical research, particularly in countries like China, Japan, India, and South Korea. The growing patient pool and rising prevalence of chronic diseases are also stimulating demand for advanced therapies requiring IVT templates.
- Latin America: Shows nascent but steady growth, primarily influenced by increasing investments in healthcare infrastructure and a growing focus on biotechnology research in countries like Brazil and Mexico. International collaborations and efforts to develop local pharmaceutical capabilities are contributing to the demand for IVT templates.
- Middle East and Africa (MEA): Currently represents a smaller market share but is expected to witness gradual growth. This growth is driven by improving healthcare systems, increasing awareness of advanced therapies, and strategic investments by governments in developing research and manufacturing capabilities in life sciences, particularly in regions like Saudi Arabia and the UAE.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the In-vitro Transcription Templates Market.- Thermo Fisher Scientific
- Promega Corporation
- New England Biolabs
- Merck KGaA
- Danaher Corporation
- Bio-Rad Laboratories
- Roche Diagnostics
- Lonza Group
- Aldevron (Danaher Company)
- Genscript Biotech Corporation
- Sartorius AG
- Takara Bio Inc.
- Transcenta Holding Limited
- StemRNA Therapeutics Inc.
- Twist Bioscience Corporation
- Creative Biogene
- Cytiva (Danaher Company)
- Sino Biological Inc.
- OriGene Technologies
- VectorBuilder Inc.
Frequently Asked Questions
What are in-vitro transcription templates used for?
In-vitro transcription templates are DNA molecules used as blueprints to synthesize specific RNA sequences outside of living cells. They are crucial for producing messenger RNA (mRNA) for vaccines and therapies, gene editing components, functional RNAs, and for basic research purposes.
What is the difference between linearized plasmid DNA and PCR-amplified DNA templates?
Linearized plasmid DNA templates are typically derived from plasmid vectors that have been cut with restriction enzymes to create a linear DNA molecule. PCR-amplified DNA templates are generated by polymerase chain reaction, offering a faster way to obtain a specific DNA fragment, often used for quick screening or when plasmid cloning is not feasible.
Which factors are driving the growth of the In-vitro Transcription Templates Market?
The market is primarily driven by the escalating demand for mRNA-based vaccines and therapeutics, advancements in gene therapy and gene editing technologies, increasing investments in life science R&D, and the growing adoption of personalized medicine approaches globally.
What impact is AI expected to have on IVT template development?
AI is anticipated to significantly optimize IVT template design, predict optimal RNA sequences for improved stability and function, automate synthesis and quality control processes, and accelerate the discovery of novel RNA-based products through advanced data analysis and predictive modeling.
Who are the major end-users of in-vitro transcription templates?
The major end-users include biotechnology and pharmaceutical companies focused on drug and vaccine development, academic and research institutes conducting fundamental biological studies, contract research organizations (CROs), and diagnostic laboratories developing molecular assays.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
